Creative Biolabs has been an innovative pioneer in the in vitro diagnostic (IVD) industry in the field of numerous diseases and infections. Our scientific staff has extensive IVD antibody development experience and offers clients the opportunity to collaborate on the development of valuable antibodies for diagnostic use to support clinical diagnosis. Particularly, we provide IVD antibody development services with high quality and low cross-reactivity for the detection of influenza A and B viruses.
Introduction of Influenza A & B
Influenza is an acute respiratory illness that has been recognized since the 16th century and spreads rapidly through communities in outbreaks. Two forms of influenza occur globally: epidemic influenza caused by influenza A and B viruses and sporadic pandemics caused by influenza A virus. Influenza viruses are enveloped particles that contain a single-stranded, segmented RNA genome. Influenza A and B viruses possess eight gene segments, which encode at least 17 proteins. The haemagglutinin protein mediates binding of influenza virus to its receptors, sialyloligosaccharides on the host cell. Human influenza viruses preferentially bind to α2,6-linked sialyloligosaccharide receptors, which predominate in the human upper respiratory tract, whereas avian influenza viruses bind to α2,3-linked sialyloligosaccharide receptors, which are more prevalent in the lower respiratory tract.
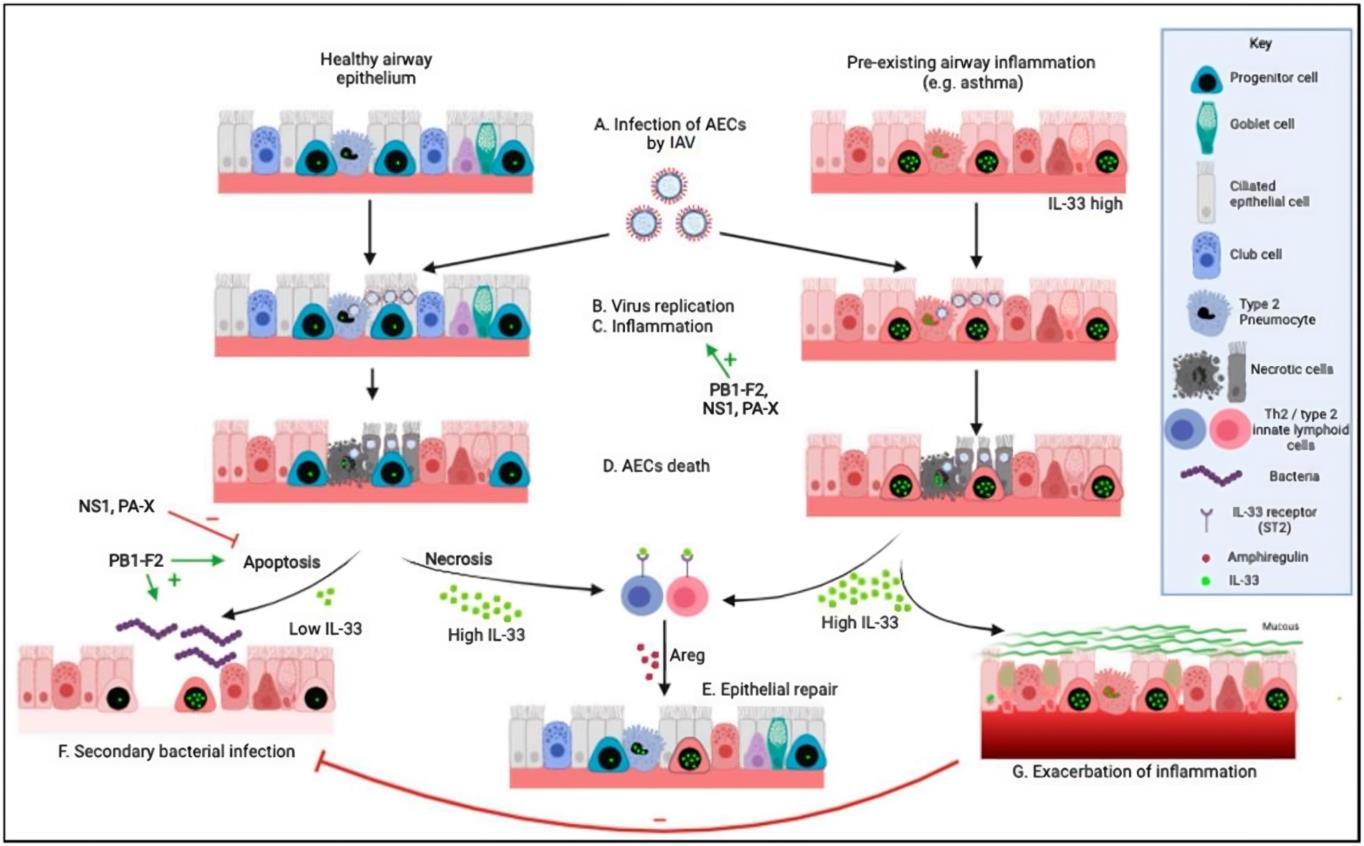 Fig.1 Effects of influenza A virus on the respiratory epithelium.1
Fig.1 Effects of influenza A virus on the respiratory epithelium.1
Clinical diagnosis of influenza A and B is difficult because symptoms range in severity and overlap with those caused by other respiratory viruses. Currently, used laboratory diagnostic methods, including viral culture, antigen detection, nucleic acid testing, and serological testing, have different sensitivity, advantages, and limitations. Here we describe two commonly used methods:
- Viral Culture
Viral culture methods, including both conventional and rapid viral culture, are highly sensitive and specific. They are usually performed in commercially supplied cells such as primary Rhesus monkey kidney (pRhMK) cells and Madin-Darby canine kidney (MDCK) cells. The turnaround time of traditional viral culture is 3-10 days, making the results not available in time to inform clinical decision making. The rapid viral culture method is much quicker but is less sensitive than the traditional one.
- Antigen Detection Assays
Antigen detection assays mainly include rapid immunoassays and direct immunostaining. Rapid immunoassays for detecting and distinguishing influenza A and B are performed with commercially supplied kits. These kits contain all of the required materials and reagents including capture antibodies immobilized on chromatographic paper, detection antibody (anti-influenza antibody) conjugates with particles for visualization, and other reagents. Similarly, the immunostaining assay employs commercial immunofluorescence antibody kits for influenza antigen detection or individual anti-influenza antibodies and conjugates. These methods have a rapid turnaround time and are simple to conduct.
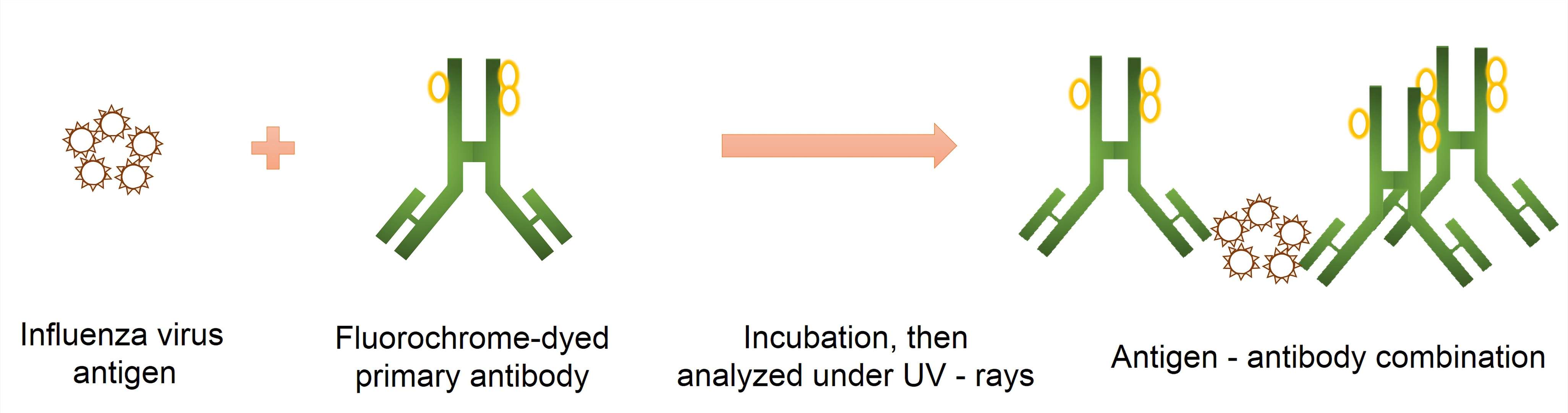 Fig.2 Schematic presentation of the direct immunofluorescence antibody test for diagnosis of influenza A. (Budama-Kilinc, Y., 2016)
Fig.2 Schematic presentation of the direct immunofluorescence antibody test for diagnosis of influenza A. (Budama-Kilinc, Y., 2016)
At present, although a number of commercial kits and anti-influenza antibodies and conjugates are available for the detection of influenza virus A and B in the cells present in respiratory secretions, the availability of testing materials that are subtype-specific is poor. Reagents and kits with high sensitivity and specificity are urgently needed to aid in clinical diagnosis and decision making.
Creative Biolabs is aiming at offering affordable and reliable in vitro diagnosis (IVD) antibody development services to clients globally. We provide customized solutions and flexible approaches for the purpose of satisfying every specific research requirement. Please contact us to discuss your project in detail and experience the value of our expert services.
References
- Rozario, Christoforos, et al. "Could Interleukin-33 (IL-33) Govern the Outcome of an Equine Influenza Virus Infection? Learning from Other Species." Viruses 13.12 (2021): 2519. Distributed under Open Access license CC BY 4.0, without modification.
- Budama‐Kilinc, Yasemin, and Rabia Cakir‐Koc. "Influenza diagnosis with a specific emphasis on the M2e antigen as a diagnostic tool." Steps Forwards in Diagnosing and Controlling Influenza (2016). Distributed under Open Access license CC BY 3.0, without modification.
For Research Use Only.