The development of advanced antibody technology has significantly facilitated the production of in vitro diagnostic (IVD) antibodies and has provided the means for developing a number of highly specific and reproducible immunoassays, such as ELISA, immunofluorescent-antibody assays, and immunohistochemistry for rapid and accurate diagnosis of an extensive list of infectious diseases. With years of experience in the field of antibody development, Creative Biolabs is capable of providing high-quality IVD antibody development services against different biomarkers associated with SARS coronavirus infection.
Introduction of SARS Coronavirus
The coronaviruses (order Nidovirales, family Coronaviridiae, genus Coronavirus) are a diverse group of large, enveloped, positive-stranded RNA viruses that cause respiratory and enteric diseases in humans and other animals. There are three groups of coronaviruses, of which groups 1 and 2 contain mammalian viruses, whereas groups 3 contain only avian viruses. Severe acute respiratory syndrome (SARS), a viral respiratory illness caused by a coronavirus, called SARS-associated coronavirus (SARS-CoV), has caused serious worldwide epidemics in 2003. Since then, there is growing demand for diagnostic tools for early, accurate, and safe diagnosis of SARS-CoV. Currently, there are two commonly used methods for the diagnosis of a SARS case, namely PCR and serological methods. Early disease is best detected by polymerase chain reaction (PCR) based tests, whilst detection of specific antibodies is the preferred diagnostic approach after 10 days of the onset of symptoms.
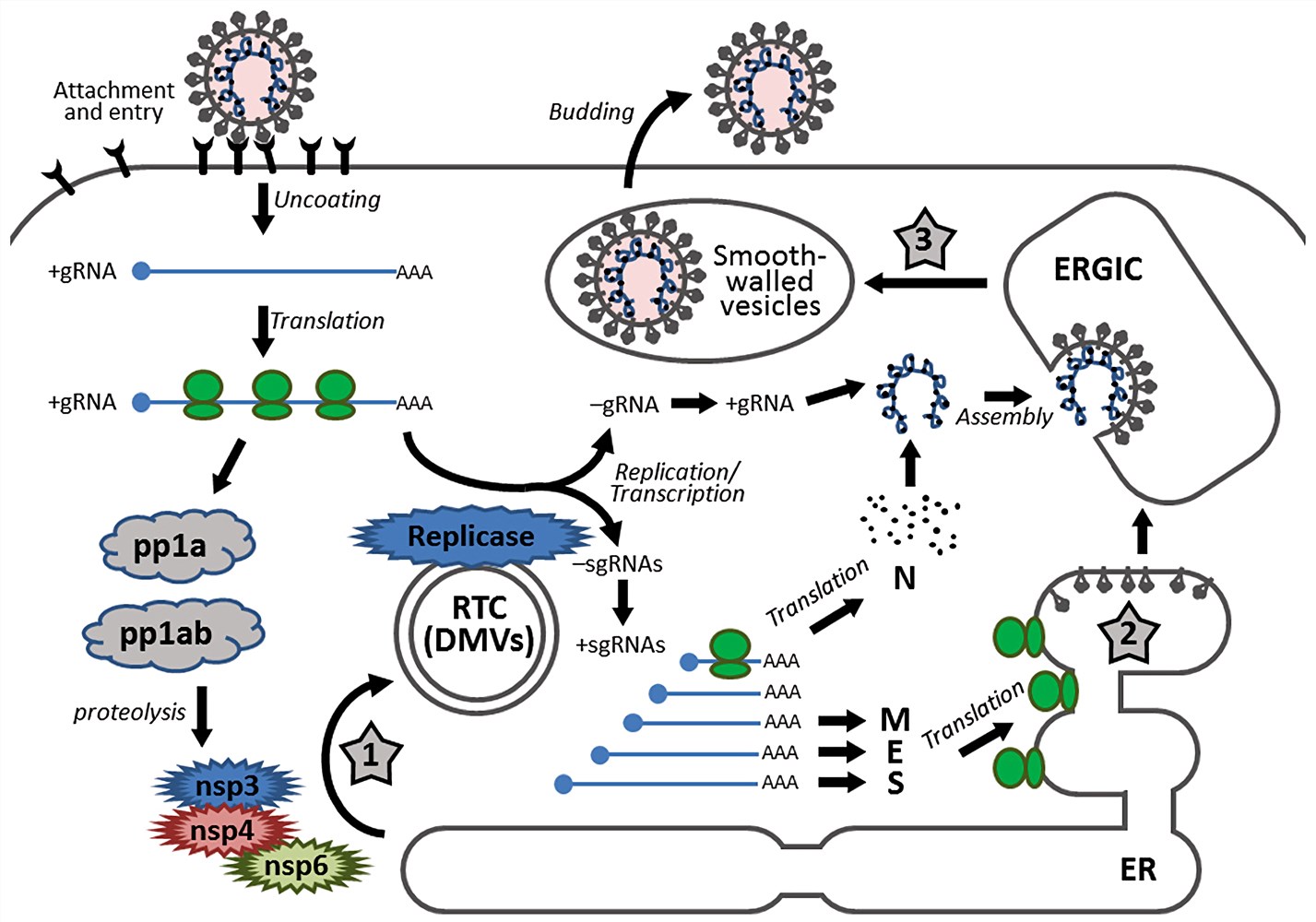 Fig.1 Schematic diagram showing the replication cycle of coronavirus. (Fung, T. S., 2014)
Fig.1 Schematic diagram showing the replication cycle of coronavirus. (Fung, T. S., 2014)
Polymerase Chain Reaction (PCR)
The PCR allows for direct detection of SARS-CoV genetic material in various patient specimens, such as respiratory, secretions, blood, stools, or body tissues. The World Health Organization (WHO) recommends that at least two different clinical specimens (e.g., nasopharyngeal and stool) or the same clinical specimen collected on two or more days during the course of the illness or two different assays or repeat PCR using the original clinical sample on each occasion of testing. The specificity of PCR tests for SARS is excellent and sensitivity can be greatly increased if multiple specimens are tested.
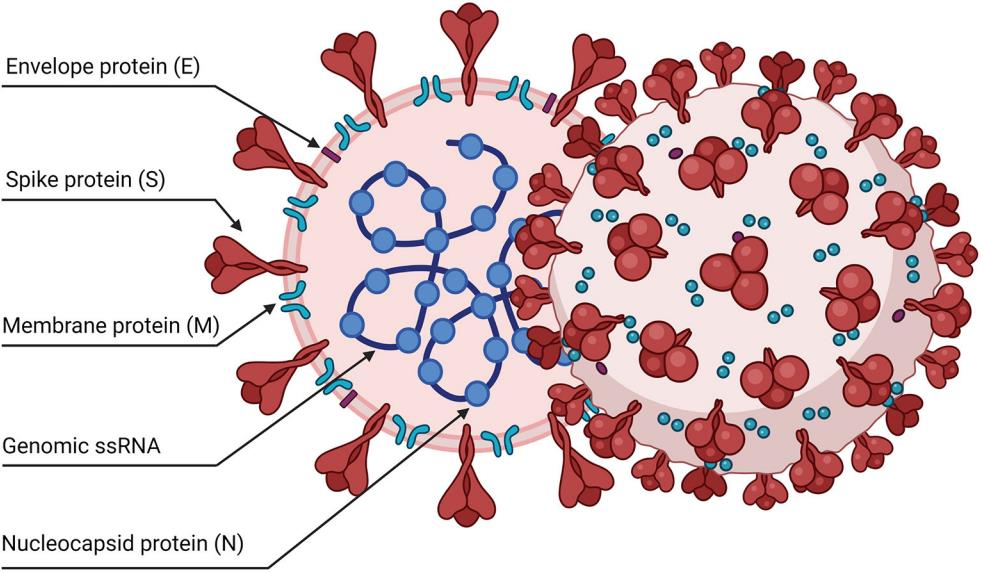 Fig.2 Schematic representation of the SARS-CoV-2 viral particle.2
Fig.2 Schematic representation of the SARS-CoV-2 viral particle.2
Serological Testing
Antibodies against SARS-CoV become detectable with high sensitivity at about 10 days after onset of infection. The determination of antibodies in the specimens typically uses methods including enzyme-linked immunosorbent assay (ELISA), immunofluorescence assays (IFA), and the immunochromatic test (ICT), among which the ELISA and IFA are more reliable due to their high sensitivity and specificity. A negative antibody test on acute serum followed by a positive antibody test on convalescent serum or four-fold or greater rise in antibody titer between acute and convalescent phase sera tested in parallel is recommended by WHO for confirmation of suspected cases.
The laboratory diagnosis of any infectious disease often needs the demonstration of the causative organism or a specific antibody. As a result, antibodies and in particular antigen-specific monoclonal antibodies, thanks to their high specificity, can be very useful in the early diagnosis of infectious diseases. At Creative Biolabs, you can find the most comprehensive first-rate IVD antibody development services for diversified diagnostic uses. Contact us to discuss your specific requirements.
References
- Fung, To S., and Ding X. Liu. "Coronavirus infection, ER stress, apoptosis and innate immunity." Frontiers in microbiology 5 (2014): 92793. Distributed under Open Access license CC BY 4.0, without modification.
- Pizzato, Massimo, et al. "SARS-CoV-2 and the host cell: a tale of interactions." Frontiers in Virology 1 (2022). Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.