With wealthy experience in the in vitro diagnostic (IVD) immunoassay development, Creative Biolabs is proud to offer customized immunoassay development services targeting potential autoantibody biomarkers, such as UH-RA.14, to improve the diagnosis of both early and seronegative rheumatoid arthritis (RA).
The Role of Biomarkers in RA
As a chronic inflammatory joint disease, RA can cause cartilage and bone damage as well as disability. Early diagnosis plays a key role in optimal therapeutic success, particularly in patients with well-characterized risk factors for poor outcomes such as high disease activity, presence of autoantibodies, and early joint damage. Biomarkers are integral to disease stratification and hence targeted therapy. They are potential in the transformation of RA management by enabling not only early diagnosis but also assessment and prediction of disease severity. In addition, they can also be utilized in the selection of therapy, and monitoring of response to therapy. Autoantibodies are among the most important biomarkers of RA and at present, none of the candidate genetic, cellular, or molecular biomarkers has yet surpassed the clinical value of RA-specific autoantibodies, including rheumatoid factor (RF) and anti-citrullinated protein autoantibodies (ACPA).
UH-RA.14 Autoantibody for RA Diagnosis
Although RA diagnostics has greatly improved using current RF and ACPA biomarkers, they are absent in one-third of the RA patients and even more in early disease. Studies have reported the discovery of autoantibodies to novel Hasselt University (UH) peptides and their potential in early RA diagnosis and RF/ACPA-seronegative RA. UH-RA.14 is one of the four novel peptides (UH-RA.1, UH-RA.9, and UH-RA.21). Results showed that the detection of autoantibodies targeting UH-RA.14 with peptide enzyme-linked immunosorbent assay can indicate reactivity in early disease, together with the other three biomarkers.
IVD Immunoassay Development Services Provided by Creative Biolabs
Immunoassays, such as ELISA, are based on the specific recognition between the analytes and the pre-coated antigen or antibodies. Development of immunoassays can be difficult, with challenges including establishing method selectivity, specificity and range of quantitation as a result of nonspecific background signal, matrix interference, lack of linearity and antibody interference. Creative Biolabs has extensive experience in immunoassay method development with expertise in bioconjugate design and synthesis, antibody generation, recombinant protein expression, as well as a wide range of immunodiagnostic test development. We work with our clients to develop assays that exactly meet their specific research and manufacturing requirements. Our long-standing know-how in this field enables us to make the most out of any immunoassay platform. For more information please click the links below:
- IVD Antibody Development
- Antibody Pair Development
- Antibody & Protein Conjugation
- IVD Immunoassay Development
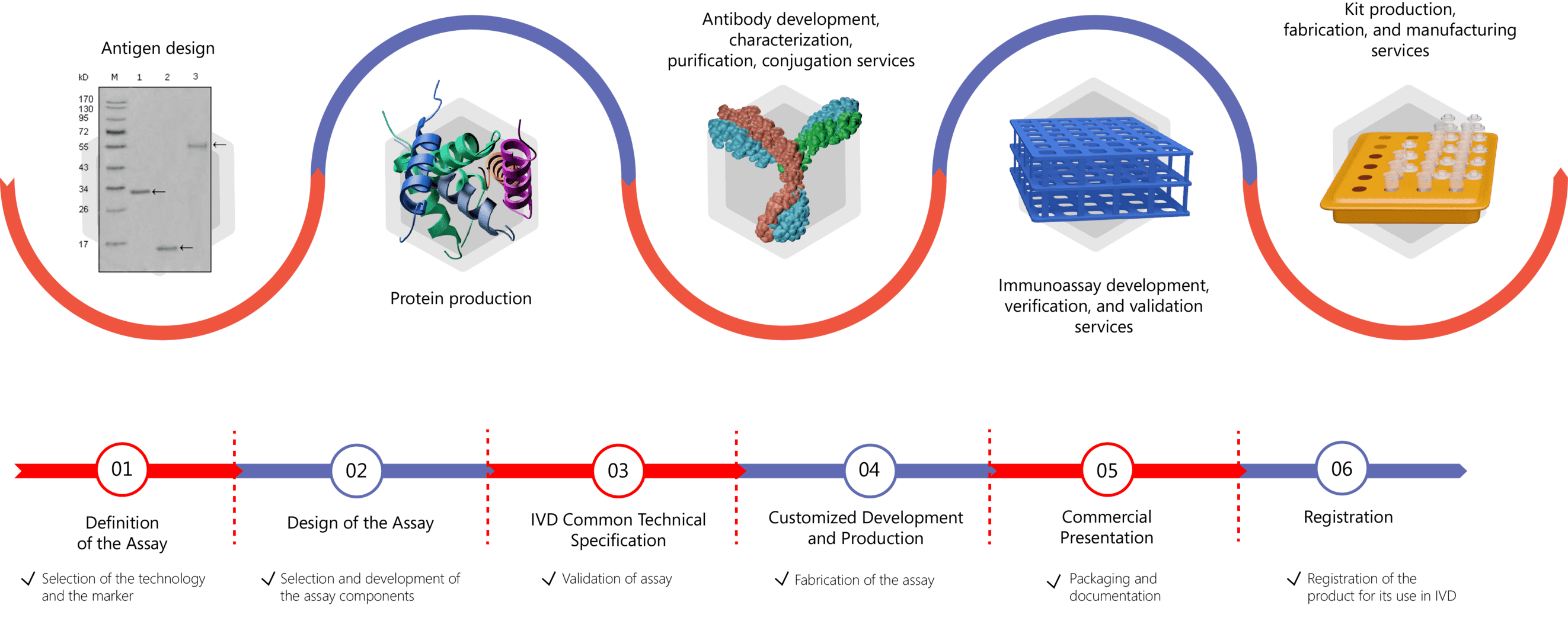
We know that high-quality antigens are of great importance for ensuring the sensitivity and specificity of diagnostic kits to detect autoantibodies in patient serum. Creative Biolabs is equipped with expertise in the production and purification of autoimmune antigens from native sources. Please feel free to contact us for more information if you are interested in our services.
For Research Use Only.