With wealthy experience in the in vitro diagnostic (IVD) immunoassay development, Creative Biolabs is proud to offer customized immunoassay development services targeting potential autoantibody biomarkers, such as UH-RA.9, to improve the early diagnosis of rheumatoid arthritis (RA).
Early Diagnosis of RA
Early diagnosis and treatment have been recognized as essential to improving clinical outcomes in patients with early RA. However, diagnosis is somewhat difficult in the early stages of the disease because the diagnostic criteria were developed from data obtained in patients with established rheumatoid arthritis and therefore are not readily applicable. Finding the ultimate biomarker for RA or rheumatoid disease (PRD) is like a proverbial pot of gold at the end of a rainbow – except there’s no rainbow. If there is one essential biomarker that could lead to tests that indicate who has the disease and how active it is while providing clues to a cure. Meanwhile, people living with PRD suffer long delays in diagnosis and difficulties obtaining adequate treatment.
UH-RA.9 Autoantibody for RA Diagnosis
Recent researches report that autoantibodies against novel UH-RA peptides (UH-RA.1, UH-RA.9, UH-RA.14, and UH-RA.21) have been identified as candidate biomarkers for patients with RA) who are seronegative for the current diagnostic markers RF (rheumatoid factor) and ACPA (anticitrullinated protein antibodies). The detection of antibody reactivity against these candidate biomarkers in 37% of early and 26% of seronegative RA patients implies that these biomarkers can be of additional value to the current diagnostic biomarkers for RA, with most promising results for UH-RA.9. These biomarkers may, therefore, contribute to an improved early diagnosis of RA. Significant associations with inflammatory factors and disease activity indicate an important prognostic potential as well.
IVD Immunoassay Development Services Provided by Creative Biolabs
Immunoassays, such as ELISA, are based on the specific recognition between the analytes and the pre-coated antigen or antibodies. Development of immunoassays can be difficult, with challenges including establishing method selectivity, specificity and range of quantitation as a result of nonspecific background signal, matrix interference, lack of linearity and antibody interference. With extensive experience in immunoassay development, Creative Biolabs offers services including but not limited to recombinant protein expression, antibody development, protocol establishment, assay validation, and manufacturing. Our professional project management team assists you throughout the entire course of a project. For more information please click the links below:
- IVD Antibody Development
- Antibody Pair Development
- Antibody & Protein Conjugation
- IVD Immunoassay Development
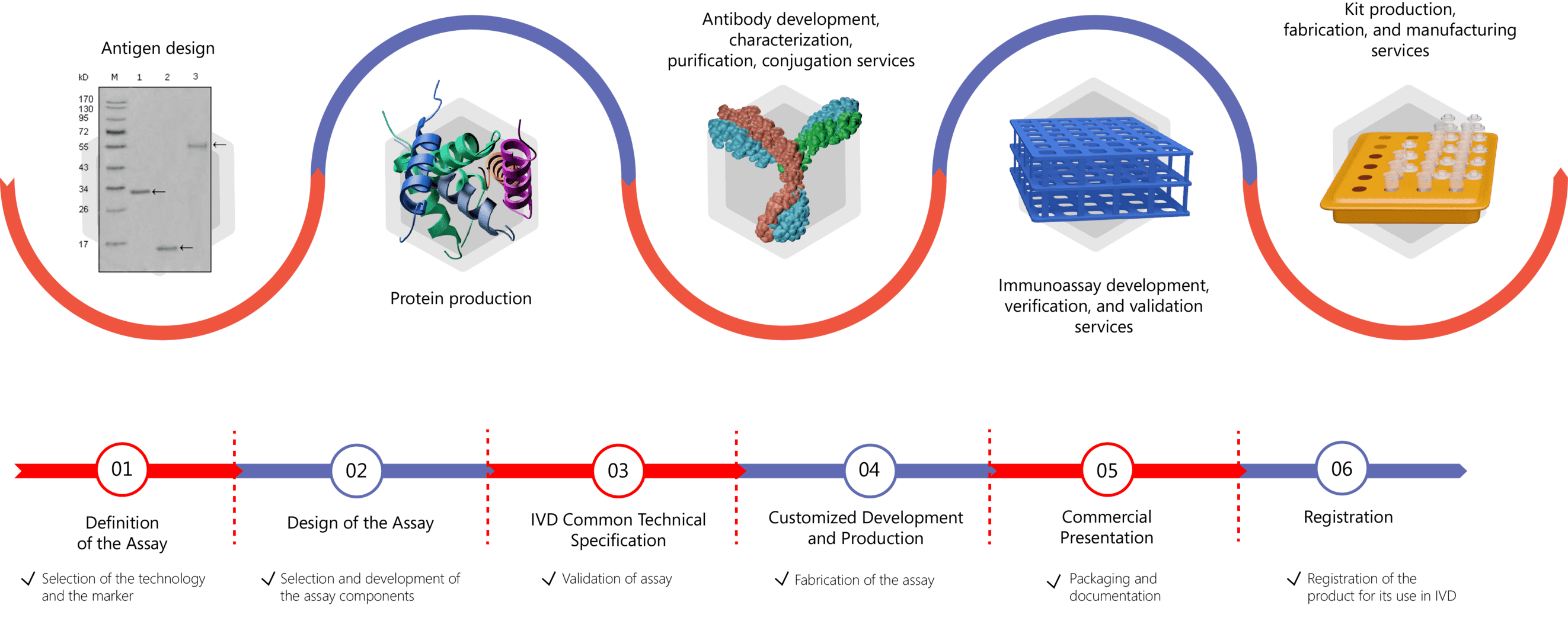
High-quality antigens are of great importance for ensuring the sensitivity and specificity of diagnostic kits to detect autoantibodies in patient serum. Creative Biolabs is equipped with expertise in the production and purification of autoimmune antigens from native sources. Please feel free to contact us for more information if you are interested in our services.
For Research Use Only.