Creative Biolabs is an expert in vitro diagnostic (IVD) antibody developer and manufacturer, providing antibodies intended for in vitro diagnosis of a wide range of viral infections, such as Zika Virus. With industry-leading antibody development skills and a well-established reputation in custom development, we are a preferred and trusted supplier of IVD antibodies with superior quality.
Introduction of Zika Virus
Zika virus (ZIKV) is a member of the virus family Flaviviridae. It is transmitted primarily by Aedes mosquitoes. ZIKV was first identified incidentally in 1947 by way of tree canopy surveillance among nonhuman primates in the Zika forest of Uganda. People with Zika virus disease can exhibit symptoms such as mild fever, skin rash, conjunctivitis, muscle and joint pain, malaise or headache. There is currently no vaccine available, but acetaminophen and rest may help with the symptoms.
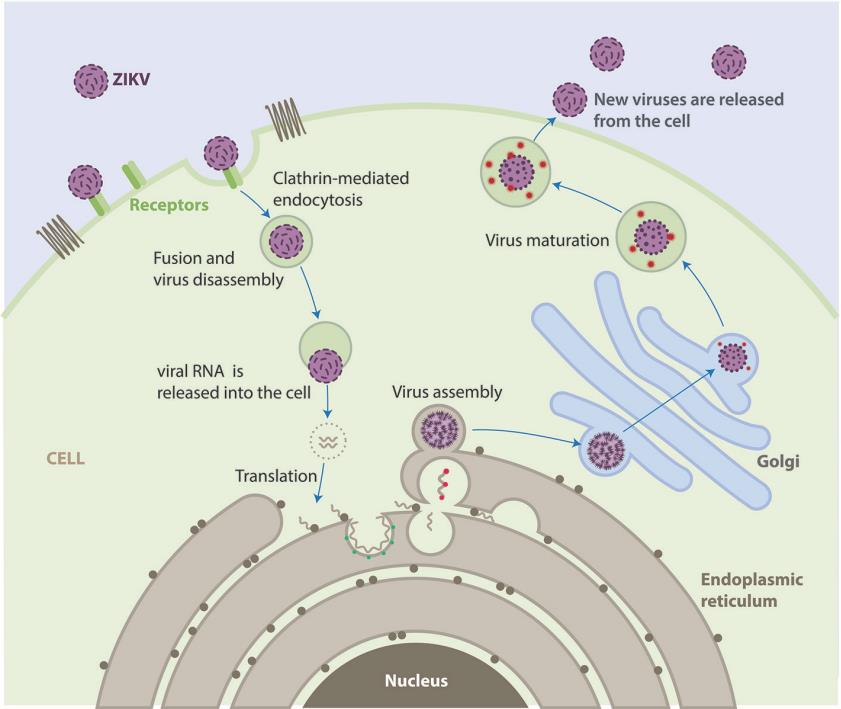 Fig.1 Life cycle of ZIKV.1
Fig.1 Life cycle of ZIKV.1
Laboratory Diagnosis of Zika Virus
Diagnostic tests to confirm suspected exposure include the molecular detection of ZIKV RNA by reverse transcriptase PCR and serology.
- RT-PCR – ZIKV RNA is usually detectable in serum by RT-PCR within 2 days of symptoms onset and up to 7 days after symptom onset. As a result, World Health Organization (WHO) recommends RT-PCR to be used in patients presenting with onset of symptoms <7 days. The diagnostic sensitivity of RT-PCR can be influenced by many factors, including specimen source, duration of illness, specimen collection and transport and so forth. But if the test is well-designed, it will be highly-specific and will not cross-react with other flaviviruses.
- Serology – ZIKV-specific IgM and neutralizing antibodies typically develop toward the end of the first week of illness, but may take up to 2 weeks. The level of IgM generally continues to be detectable for 12 weeks after symptom onset. The most widely used test for serologic diagnosis of ZIKV infection is the IgM antibody capture (MAC)-enzyme-linked immunosorbent assay (ELISA) with the confirmatory plaque reduction neutralization test (PRNT). Other tests are also used such as the immunofluorescence assays (IFA). Serological testing for ZIKV should only be conducted by laboratories with experience in performing flavivirus serology.
 Fig.2 Strategy for developing liposome immunoassay-based fluorescence immunoassay for the detection of ZIKV. (Shukla, S., 2016)
Fig.2 Strategy for developing liposome immunoassay-based fluorescence immunoassay for the detection of ZIKV. (Shukla, S., 2016)
In Vitro Diagnostics (IVDs)
There is increasingly strong interest and need for rapid and simple to use IVDs for ZIKV infections. Numerous studies have concentrated on the development of immunoassays that can be used to detect ZIKV infections, such as liposome-based assays. To date, however, few commercially available ZIKV IVDs have been developed and undergone regulatory assessment of quality, safety, or performance. To promote the development of IVD kits and to help clinical diagnosis of ZIKV, Creative Biolabs is providing first-class IVD antibody development services to both domestic and overseas clients. Moreover, Services provided here can be tailored to meet the specific needs of our clients.
Contact us to discuss your specific requirements and experience the great value of our expert services.
References
- Acosta-Ampudia, Yeny, et al. "Autoimmune neurological conditions associated with Zika virus infection." Frontiers in molecular neuroscience 11 (2018): 116. Distributed under Open Access license CC BY 4.0, without modification.
- Shukla, S., (2016). “Rapid detection strategies for the global threat of zika virus: current state, new hypotheses, and limitations.” Frontiers in Microbiology, 7(540). Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.