To facilitate the development of higher-quality in vitro diagnostic (IVD) products, Creative Biolabs offers specialized discovery and development services for diagnostic antibodies. These services span diverse disease areas, including cancer, autoimmune disorders, infectious diseases, etc. Our advanced technological platform and experienced scientific team ensure meticulous execution of each process, from antigen preparation to custom antibody production.
To Know Our In Vitro Diagnostics
IVD products, encompassing reagents, kits, antibodies, and calibrators, are crucial for rapid, convenient, and accurate diagnosis and monitoring of human diseases. Among these, IVD antibodies are indispensable components in antibody-based kits and diagnostic assays. The initial and most critical step in developing reliable immunodiagnostic assays is obtaining antibodies with high affinity and specificity for relevant biological markers. The continuous emergence of new technologies has not only enhanced the sensitivity, accuracy, and reliability of IVD but has also significantly reduced associated costs and development timelines.
To Know Our Main IVD Antibody Development Services
The main IVD antibody development services include:
|
|
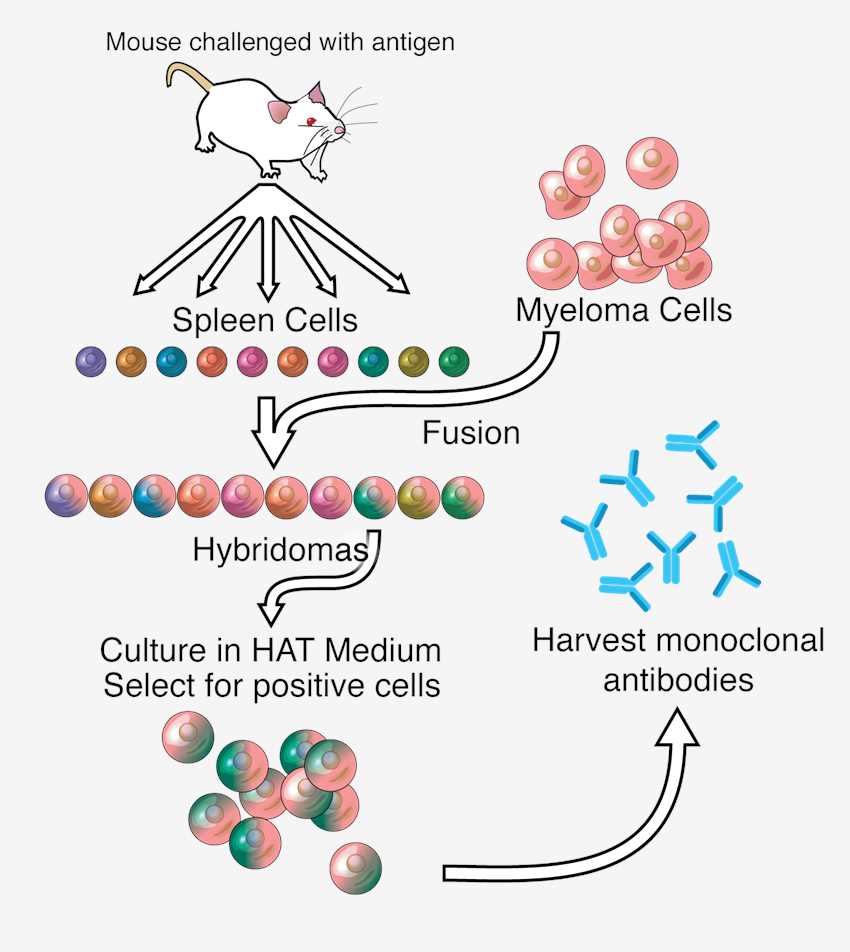
Monoclonal Antibody DevelopmentMonoclonal antibodies have always been the protagonists of basic research, therapeutics, and diagnostics. With decades of experience, Creative Biolabs offers one-stop monoclonal antibody development services using a variety of methods, such as hybridomas, phage display technologies, recombinant expression, and single B-cell technology. Our monoclonal antibody development service is flexible, cost-effective, and time-saving, and has won a good reputation during these decades. |
|
|
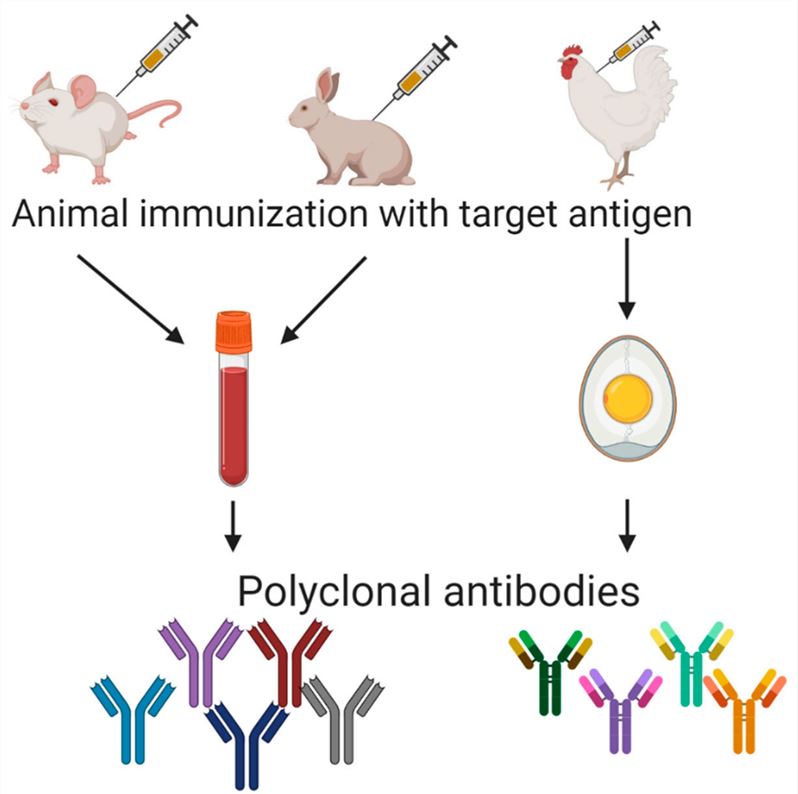
Polyclonal Antibody DevelopmentPolyclonal antibodies recognize multiple epitopes on an antigen and have relatively lower cost and shorter lead-time in preparation. Creative Biolabs offers high-quality polyclonal antibody development services, covering a variety of species, including rabbits, goats, chickens, and more. And each polyclonal antibody development project is tailored to your specific needs. |
|
|
Recombinant Antibody DevelopmentCreative Biolabs provides recombination antibody services covering from gene synthesis to antibody purification. Related antibody engineering services, such as antibody affinity maturation, antibody chimerization, antibody humanization, isotype switching, antibody reformatting, etc., are also within our capabilities. |
|
|
Antibody Pairs DevelopmentAntibody pairs, consisting of capturing antibodies and detecting antibodies, are two different antibodies generated against different epitopes or binding regions on one target. The matched antibody pairs are usually applied for antigen or biomarker detection and enzyme-linked immunosorbent assays. In addition to a wide range of off-the-shelf antibody pair sets, Creative Biolabs provides development services for antibody pairs in different formats, including mAb-mAb, mAb-pAb, and pAb-pAb. |
To Know Our IVD Antibody Development Process
Our streamlined and customizable IVD antibody development process ensures the generation of high-quality antibodies tailored to your specific diagnostic needs:
This crucial first step involves meticulous immunogen design. We select optimal antigen sequences, considering immunogenicity and cross-reactivity. The chosen protein expression system (bacterial, yeast, insect, mammalian) ensures purified antigen integrity and functionality for effective immunization.
Purified immunogen is introduced into selected animal hosts (mice, rats, rabbits, etc.) to stimulate a potent immune response. Our optimized immunization protocols, including adjuvant selection and precise dosing, elicit strong, specific antibody titers tailored to project requirements.
Leveraging cutting-edge technologies, we employ diverse strategies for antibody generation. Monoclonal antibodies use traditional hybridoma technology. For recombinant antibodies, advanced techniques like single B cell cloning or phage display enable rapid selection of highly specific fragments. This ensures customized monoclonal or polyclonal antibodies perfectly suited for your application.
A robust screening process identifies antibodies with desired binding characteristics. We use sophisticated assays like ELISA, Western blot, and cell-based methods to evaluate specificity, affinity, and lack of cross-reactivity. This meticulous selection ensures only the most promising candidates proceed.
Candidate antibodies undergo rigorous purification to achieve high purity essential for diagnostic applications. Our methods include salting-out and various chromatography techniques (gel column, ion exchange, immunoaffinity). This ensures the final antibody product is pure and suitable for direct use in IVD assays.
Beyond basic purification, we offer advanced antibody optimization and modification to enhance performance in specific IVD formats. This includes precise labeling with detection tags (enzymes, fluorophores, biotin, gold nanoparticles) and fragmentation (Fab, scFv) for optimal signal-to-noise ratios and stability.
Comprehensive functional validation confirms antibody performance in its intended diagnostic context. We rigorously verify efficacy through a suite of assays: Western blot, ELISA, Immunohistochemistry (IHC), and flow cytometry. This ensures reliable and accurate performance within the specific IVD assay format.
Upon successful validation, we offer scalable antibody production, from small-scale R&D to large-scale manufacturing. Our processes are meticulously controlled for batch-to-batch consistency, high yield, and adherence to stringent quality standards, providing a reliable supply of high-performance IVD antibodies.
To Know Our IVD Antibody Development Advantages
Broad Biomarker Targeting
Custom IVD antibody development targets a wide array of disease biomarkers, including autoimmune diseases, cancers, infectious agents, and cardiovascular conditions, ensuring comprehensive diagnostic reach.
Diverse Antibody Technologies
Capabilities encompass multiple antibody technologies. High-specificity monoclonal, robust polyclonal, and versatile recombinant antibodies (via hybridoma, phage display, or single B cell) are produced, allowing optimal method selection.
Integrated Ancillary Expertise
Beyond core antibody generation, extensive ancillary expertise is available. This covers advanced purification, comprehensive characterization (specificity, affinity, stability), and rigorous functional validation across diverse IVD assay formats, providing a complete solution.
Fully Customizable Experimental Design
Recognizing unique diagnostic projects, fully customizable experimental designs are offered. This flexible approach tailors every development step to specific requirements, timelines, and budgets, maximizing efficiency and success.
Commitment to Quality and Compliance
Every developed antibody undergoes stringent quality control. Processes are designed with future regulatory submissions in mind, delivering high-quality, reliable antibodies meeting industry standards.
Q&A
-
Q: What factors are most critical in determining the success of an IVD antibody development project?
A: The success of an IVD antibody development project hinges primarily on the quality of the immunogen, the specificity and affinity of the resulting antibody, and its validated performance within the intended diagnostic assay. Careful antigen design, robust screening methods, and thorough functional characterization are all indispensable for achieving a reliable and accurate diagnostic tool.
-
Q: Can you develop antibodies against challenging or low-immunogenicity targets?
A: Absolutely. Developing antibodies against difficult targets, such as small molecules, highly conserved proteins, or those with low immunogenicity, is a core strength at Creative Biolabs. We utilize specialized immunization strategies, including carrier proteins and optimized adjuvant formulations, alongside advanced recombinant antibody technologies like phage display, to overcome these challenges and successfully generate high-affinity antibodies.
-
Q: What types of antibody formats can you provide for IVD applications?
A: We offer a versatile range of antibody formats to suit various IVD applications. This includes full-length monoclonal antibodies (IgG, IgM), polyclonal antibodies, and recombinant antibody fragments such as Fab, scFv, and diabodies. We can also develop optimized antibody pairs for sandwich assays, ensuring maximum performance in your specific diagnostic platform.
-
Q: How do you achieve batch-to-batch consistency for large-scale antibody production?
A: Maintaining consistent antibody performance across different production batches is paramount for IVD products. We implement stringent quality control measures at every stage of large-scale production, from cell line stability and culture conditions to purification parameters and final product characterization. Each batch is rigorously tested for purity, concentration, specificity, and functional activity to guarantee reproducibility.
-
Q: Can you assist with the regulatory aspects of IVD antibody development?
A: While we do not directly handle regulatory submissions, our antibody development processes are designed with future regulatory compliance in mind. We adhere to rigorous quality management systems and provide comprehensive documentation for all development stages, including detailed characterization and validation data. This meticulous record-keeping supports your regulatory filings for IVD product approval.
-
Q: What type of assistance do you give once the antibody development project is completed?
A: Creative Biolabs is committed to long-term client satisfaction. After project completion, we offer ongoing support, including technical assistance for antibody integration into your assays, troubleshooting, and potential re-validation or optimization if your assay parameters evolve. We strive to be a continuous partner in your diagnostic success, ensuring the sustained performance of the antibodies we develop.
IVD antibodies are critical components in numerous diagnostic assays. Leveraging extensive experience and unparalleled expertise, Creative Biolabs offers IVD antibody development services. For generating antibodies precisely tailored to your particular requirements, please contact us for more information.
References
- Garcia-Calvo, Eduardo, et al. "From polyclonal sera to recombinant antibodies: A review of immunological detection of gluten in foodstuff." Foods 10.1 (2020): 66. Distributed under Open Access license CC BY 4.0, without modification.
- Ziraldo, Gaia, et al. "A human-derived monoclonal antibody targeting extracellular connexin domain selectively modulates hemichannel function." Frontiers in Physiology 10 (2019): 392. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.