Rapid detection of infectious diseases such as influenza, malaria, HIV, and COVID-19. Our kits also facilitate early pregnancy testing and the identification of crucial cancer biomarkers and cardiac markers, supporting timely medical interventions.
Creative Biolabs leads in in vitro diagnostics (IVD) manufacturing, specializing in antibody production and rapid kit development. We offer comprehensive lateral-flow immunochromatographic assay (LFIA) kit development services, tailored for disease screening, infection detection, and therapeutic monitoring. Our commitment is to provide bespoke, application-specific solutions aligning with each client's unique needs.
Lateral-Flow Immunochromatographic Assay
LFIA, often recognized as a lateral flow immunoassay or rapid test, represents a sophisticated yet user-friendly diagnostic platform. This solid-phase immunoassay ingeniously combines the principles of thin-layer chromatography with highly specific immune recognition reactions. A liquid sample, propelled by capillary action across a porous membrane strip, interacts with dried reagents, including antibodies conjugated to visible labels such as colloidal gold, latex beads, or fluorescent quantum dots. This interaction culminates in the formation of distinct colored lines, providing a rapid and clear visual indication of the target analyte's presence or absence within minutes.
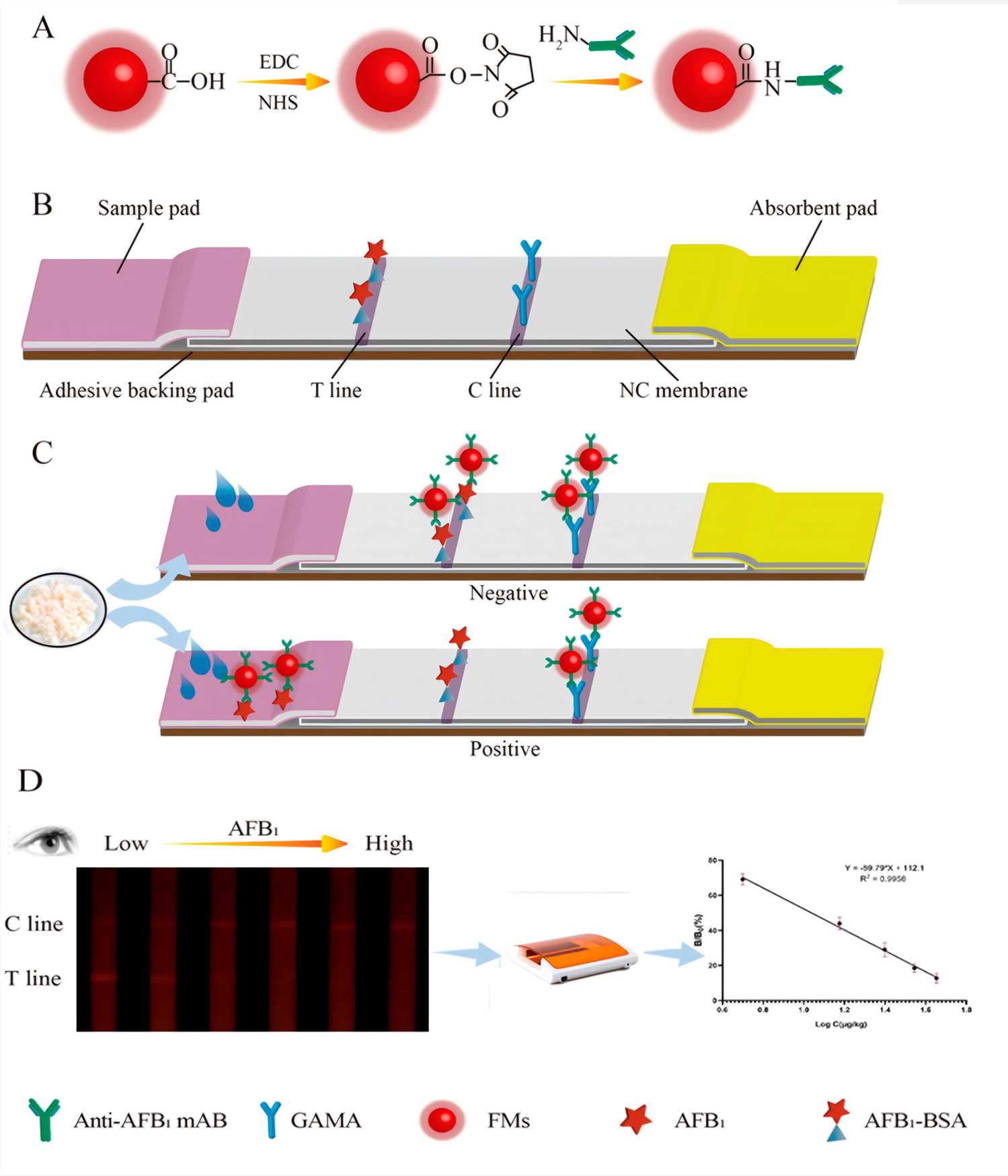 Fig.1 The structure chart and principle of FM-LFIA test strip.1,3
Fig.1 The structure chart and principle of FM-LFIA test strip.1,3
Advantages of Our LFIA Platform
LFIA technology offers a compelling suite of advantages that have revolutionized point-of-care diagnostics:
- Rapid Results: LFIA kits consistently deliver accurate diagnostic outcomes within minutes, enabling swift decision-making in critical scenarios.
- Ease of Use: These assays are designed for simplicity, requiring minimal training and no specialized laboratory equipment, making them highly accessible in diverse settings.
- Cost-Effectiveness: Utilizing inexpensive materials and amenable to high-volume manufacturing, LFIA offers a highly economical diagnostic solution for widespread deployment.
- Portability: Compact and lightweight, LFIA devices are ideally suited for fieldwork, remote testing, and home-use applications, often without refrigeration needs.
- Versatility: LFIA platforms are adaptable for qualitative, semi-quantitative, and quantitative detection, accommodating a broad spectrum of diagnostic requirements.
Our LFIA Based Kits Development Services
At Creative Biolabs, we specialize in providing a meticulously customized LFIA-based kit development service, moving beyond generic solutions to address your precise diagnostic challenges. Our expertise encompasses the entire development lifecycle, from initial concept validation and highly specific reagent selection to advanced assay optimization and robust manufacturing scale-up. We focus on engineering high-performance kits that deliver exceptional sensitivity and specificity for your target analytes, ensuring reliable results whether for complex biological matrices or environmental samples. Our service is designed to translate your unique diagnostic vision into a high-quality, market-ready product.
Service Workflow of LFIA Based Kits Development
Our LFIA kit development projects follow a systematic workflow for optimal outcomes, commencing with an initial consultation to comprehensively understand your needs. The process includes:
We begin by collaborating to outline the specific analyte, desired performance metrics (e.g., sensitivity, specificity), and the precise application of the diagnostic.
Our team meticulously chooses high-affinity antibodies or antigens and expertly conjugates them to signal nanoparticles, optimizing for stability, consistent release, and signal intensity.
We carefully select ideal materials and apply specific pre-treatments for the sample, conjugate, and absorbent pads, ensuring consistent capillary flow and efficient reagent interaction across the nitrocellulose membrane.
This crucial phase focuses on precisely depositing capture reagents onto the test and control lines and developing proprietary buffer formulations to achieve clear signal generation and minimal background interference.
We rigorously assemble prototype devices and conduct comprehensive testing for performance, stability, and reproducibility under various conditions, identifying and resolving any issues.
Finally, upon successful validation, we transition the optimized design to high-volume production using automated processes, ensuring consistent quality and timely delivery of your finished LFIA kits.
Applications
Medical Diagnostics
Food Safety
Precise identification of foodborne pathogens like E. coli, Salmonella, and Listeria. Our solutions also detect common allergens and harmful contaminants such as aflatoxins and pesticide residues, ensuring consumer health and regulatory compliance.
Environmental Monitoring
Effective monitoring of water quality for various pollutants and detection of environmental toxins. These kits are invaluable for assessing ecological health and ensuring compliance with environmental safety standards.
Veterinary Diagnostics
Swift and accurate detection of diseases in livestock and companion animals. Our LFIA kits support animal health management, disease surveillance, and the prevention of widespread outbreaks in agricultural settings.
Published Data
1. Development of Monoclonal Antibody-Based Colorimetric Lateral Flow Immunoassay
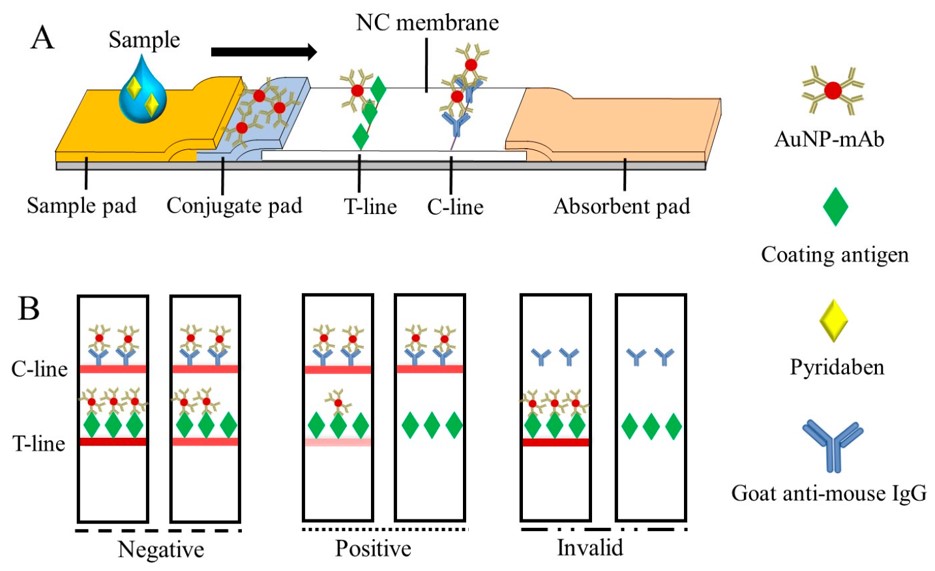 Fig.2 Generation of the colorimetric signal on the CLFIA.2,3
Fig.2 Generation of the colorimetric signal on the CLFIA.2,3
In this study, researchers first synthesized a pyridaben hapten to generate mAbs, with 6E3G8D7 demonstrating the greatest sensitivity in an indirect competitive ELISA, yielding a 50% inhibitory concentration (IC50) of 3.49 ng/mL. This mAb was then used in a colorimetric lateral flow immunoassay (CLFIA) utilizing gold nanoparticles to detect pyridaben. The CLFIA exhibited a visual detection limit of 5 ng/mL, with high specificity and outstanding accuracy across various matrices. Pyridaben concentrations in blind samples detected by CLFIA correlated well with results from high-performance liquid chromatography. Overall, the CLFIA offers a promising, dependable, and portable platform for the rapid, on-site detection of pyridaben in agricultural products and environmental samples.
2. Development of Dual-Signal Fluorescence-Enhanced Lateral Flow Immunoassay
This work presented a dual-signal lateral flow immunoassay (LFIA) featuring an improved fluorescence pattern for rapid, on-site, and ultrasensitive identification of the monkeypox virus (MPXV) antigen. The researchers developed a multilayered SiO2-Au core dual-quantum dot (QD) shell nanocomposite (SiO2-Au/DQD) as a novel nanotag, replacing traditional gold nanoparticles and QD nanobeads. This composite offered excellent stability, strong colorimetric capability, and superior fluorescence intensity. The dual-signal LFIA detected MPXV antigen (A29L) within 15 minutes, with detection limits of 0.5 ng/mL for colorimetric and 0.0021 ng/mL for fluorometric modes. The SiO2-Au/DQD-LFIA's colorimetric performance matched that of the AuNP-LFIA, but the fluorescent signal boosted sensitivity by 238-fold compared to AuNP-LFIA and 3.3-fold compared to ELISA, demonstrating its exceptional sensitivity and versatility for point-of-care MPXV testing.
Service Highlights
- Customized Solutions: We provide bespoke LFIA kit development, precisely tailored to your unique analyte, sample type, and performance requirements, ensuring optimal fit for your specific application.
- Expert Team: Benefit from our decades of specialized experience in IVD development, leveraging deep scientific knowledge and advanced technical capabilities to overcome complex challenges.
- Robust Quality Systems: Our development and manufacturing processes adhere to stringent ISO-certified and GMP-compliant quality management systems, guaranteeing the reliability and consistency of every kit.
- Accelerated Timelines: Through our systematic workflow, optimized processes, and advanced automation, we efficiently navigate development phases, significantly reducing your time to market.
FAQs
-
Q: How do you ensure the sensitivity and specificity of the developed LFIA kits?
A: Achieving optimal sensitivity and specificity involves a comprehensive strategy: selecting high-affinity antibodies or antigens for conjugation and capture. Our optimization protocols adjust reagent concentrations, buffer formulations, and membrane characteristics to maximize binding efficiency and minimize non-specific interactions. Extensive validation, including cross-reactivity and LOD determinations, verifies robust assay performance.
-
Q: Can your LFIA kits be quantitative, or are they only qualitative?
A: Our LFIA kits extend beyond qualitative results, offering semi-quantitative or fully quantitative measurements. For precise quantification, we integrate advanced signal reporters and design assays compatible with portable readers or smartphone applications. These sophisticated readers accurately measure test line intensity, enabling a direct correlation with analyte concentration for specific diagnostic requirements.
-
Q: How do you handle matrix effects and interferences in complex samples?
A: Addressing matrix effects and interferences in complex samples is pivotal. We mitigate these challenges by carefully selecting sample pad materials that filter particulates and adjust pH levels. Our proprietary buffer formulations minimize non-specific binding and counteract interfering substances. Comprehensive testing guarantees robust real-world performance.
-
Q: What quality control measures are in place during the manufacturing process?
A: Throughout manufacturing, we uphold rigorous quality control at each critical stage: raw material inspection, precise in-process checks for reagent dispensing and strip assembly, and comprehensive final product validation. Our operations adhere strictly to ISO-certified and GMP-compliant quality management systems, ensuring consistent batch reproducibility and unwavering product reliability.
-
Q: How do you ensure the scalability of LFIA kit production?
A: We guarantee LFIA kit production scalability by integrating optimized manufacturing protocols with advanced automation. Our development processes are meticulously designed with scalability as a foundational principle from inception, facilitating a seamless transition from prototypes to high-volume manufacturing. Automated dispensing, cutting, and assembly systems minimize manual intervention, ensuring consistent quality and uniform performance across large batches, effectively addressing diverse market demands.
In Creative Biolabs, we have developed high-quality LFIA-based kits for IVD. By using our IVD kits, varieties of biological specimens can be tested, involving saliva, sweat, urine, serum, plasma, whole blood, and other fluids. Moreover, further industries in which LFIA-based kits can be served that include quality control, veterinary medicine, product safety of food production, and environmental health and safety. For more detailed information, please feel free to contact us.
References
- Wang, Zifei, Pengjie Luo, and Baodong Zheng. "A rapid and sensitive fluorescent microsphere-based lateral flow immunoassay for determination of aflatoxin B1 in distillers' grains." Foods 10.9 (2021): 2109.
- Chen, He, et al. "Monoclonal antibody-based colorimetric lateral flow immunoassay for the detection of pyridaben in the environment." Biosensors 13.5 (2023): 545.
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.