Early-stage therapeutic development heavily relied on mouse models due to their efficient hybridoma production. However, pronounced human anti-mouse antibody (HAMA) responses restrict their clinical implementation despite historical prevalence.
Through years of professional service experience for our worldwide clients, Creative Biolabs is a pioneer in the in vitro diagnostic (IVD) field in the domestic and international markets. Our well-established IVD antibody development infrastructure enables multidisciplinary teams to deliver premium monoclonal antibody (mAbs) production services for diverse diagnostic and research applications. Technical proficiency combined with innovative systems guarantees the creation of antibodies demonstrating rigorous specificity and reproducibility aligned with project parameters.
Introduction
MAbs originate from a single B cell lineage and exhibit exceptional specificity through uniform epitope recognition. This singular binding capability establishes mAbs as indispensable instruments across biomedical disciplines. In contrast to polyclonal antibodies generated through multiple B cell populations that interact with varied antigenic regions, each mAb constitutes an exact molecular replica designed for exclusive interaction with defined targets. The standardized composition of mAbs proves critical for applications demanding strict reproducibility, including therapeutic agent development, high-sensitivity diagnostic testing, and molecular interaction studies. Their capacity for selective molecular engagement minimizes non-specific interactions, thereby optimizing functional precision while reducing adverse effects.
Classified by Source Species
mAbs classification systems often consider the species of origin, as the host organism critically impacts immunogenic potential during cross-species utilization. Primary source organisms we provide include:
Mouse Monoclonal Antibodies
Rat Monoclonal Antibodies
While sharing technical parallels with murine counterparts through hybridoma methodologies, rat-derived mAbs present comparable human immunogenicity limitations while demonstrating niche functional advantages in specialized diagnostic configurations.
Hamster Monoclonal Antibodies
Less frequently employed than rodent variants, these antibodies serve particular investigative roles requiring species-specific compatibility.
Chicken IgY Antibodies
Distinct from mammalian systems, chicken-produced IgY antibodies enable yolk extraction protocols – an ethically favorable alternative to mammalian blood collection that simplifies large-scale production logistics while maintaining biological functionality.
Monoclonal Antibody Technology Platform
We leverage a comprehensive suite of advanced technology platforms for mAb development:
Hybridoma Technology
Originating from Köhler and Milstein's foundational work, this conventional approach merges antigen-specific B lymphocytes with continuously proliferating myeloma cells to establish self-replicating antibody factories. The process initiates with antigen exposure in a host organism (commonly murine models), followed by spleen-derived B cell extraction. Fusion with malignant plasma cells creates durable hybrids capable of sustained mAb secretion. Post-fusion screening identifies clones demonstrating target specificity, which undergo expansion for continuous antibody harvest. Despite its historical significance, the method faces constraints, including prolonged development timelines and species-restricted antibody profiles that may lead to immunogenicity issues.
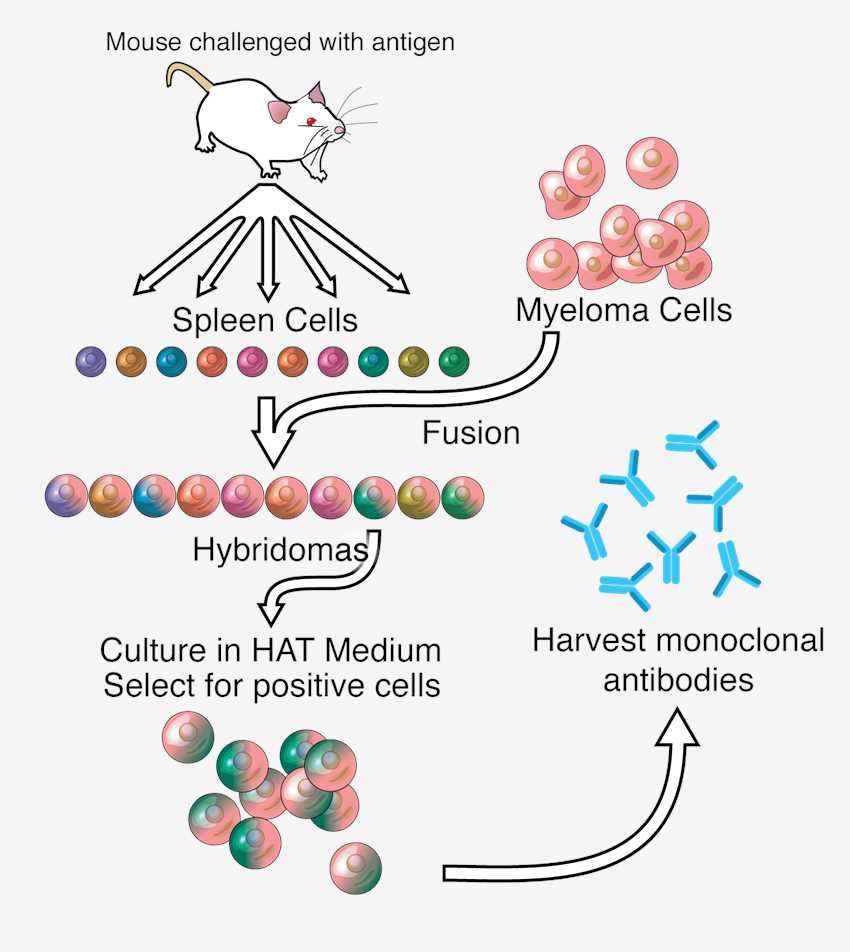 Fig.1 Overview of hybridoma technology and monoclonal antibody creation. Distributed under CC BY-SA 3.0, from Wiki, without modification.
Fig.1 Overview of hybridoma technology and monoclonal antibody creation. Distributed under CC BY-SA 3.0, from Wiki, without modification.
Phage Display Technology
This molecular screening strategy couples antibody fragment expression with bacteriophage surface presentation, enabling affinity-based identification from extensive genetic repositories. Genetic sequences encoding antigen-binding domains (e.g., Fab or scFv regions) become integrated into viral genomes, resulting in phage particles exhibiting distinct antibody variants. Iterative antigen exposure cycles progressively enrich phages demonstrating strong target binding, with subsequent isolation and genetic recovery of superior candidates. The methodology facilitates antibody development against challenging antigens with low immunogenic potential while permitting human-derived sequence recovery, thereby minimizing immunological complications during therapeutic deployment.
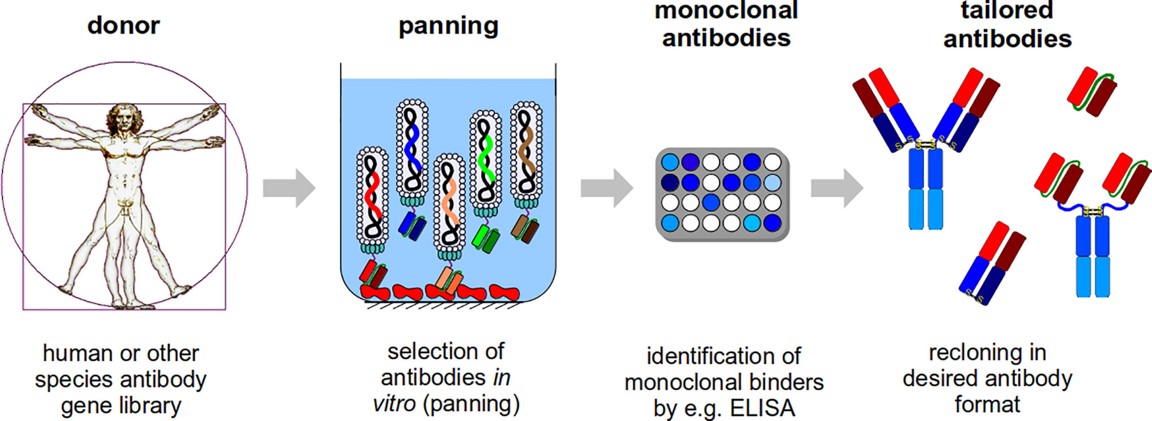 Fig.2 Schematic illustration of the mAbs generation process via phage display.1,5
Fig.2 Schematic illustration of the mAbs generation process via phage display.1,5
Single B Cell Cloning Technology
Modernized protocols circumvent traditional limitations by directly harvesting immunoglobulin genes from individual B lymphocytes, enabling comprehensive antibody diversity capture, including non-stimulated human donor repertoires. The technique isolates antibody-secreting cells from multiple biological reservoirs (peripheral circulation, lymphoid tissues, hematopoietic niches) via fluorescence-activated sorting or microfluidic devices. Following single-cell capture, reverse transcription PCR amplifies variable region sequences for subsequent recombinant expression in mammalian cell lines. This approach preserves natural antibody diversity patterns from physiological immune challenges while achieving human-compatible specificity profiles through direct clonal expansion, bypassing interspecies compatibility hurdles inherent in earlier methodologies.
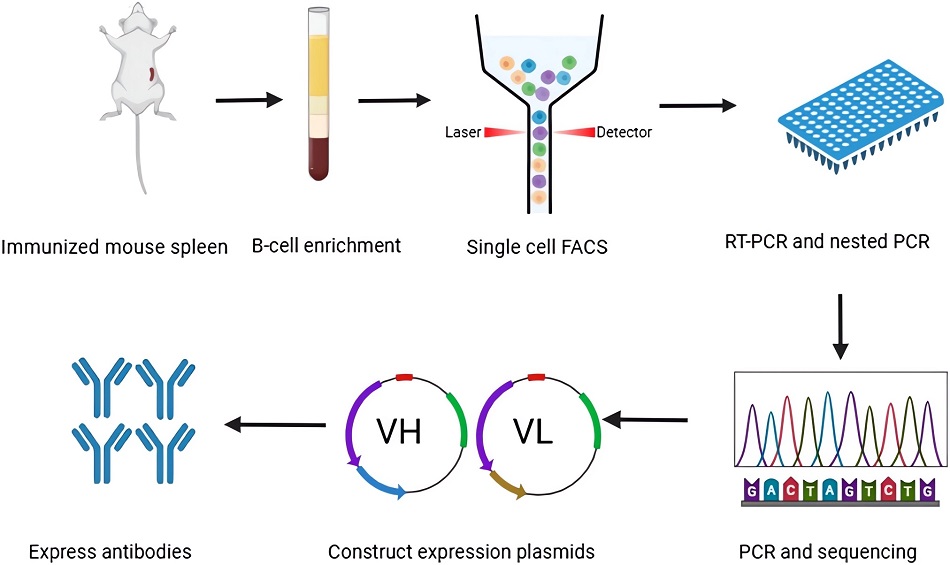 Fig.3 Schematic of the platform for generating mAbs from single B cells in mice.2,5
Fig.3 Schematic of the platform for generating mAbs from single B cells in mice.2,5
Workflow
Recognizing the distinct nature of every research initiative, our services adapt to your project's unique biological and technical demands. Core offerings include:
Antigen Selection and Preparation
Collaborative antigen selection protocols paired with customized preparation methods to enhance immunogenic potential.
Immunization Strategy Design
Species-specific immunization regimens calibrated to balance humoral response intensity with target specificity.
Hybridoma Screening Program
Multi-parametric screening matrices combining binding affinity assessments, cross-reactivity profiling, and functional validation to isolate optimal clones.
Antibody Engineering Modification
Structural modification pipelines encompassing affinity enhancement, species compatibility conversion (e.g., humanization), and functional domain optimization (Fc engineering).
Antibody Production Scale
Versatile production capabilities spanning milligram-scale exploratory batches to kilogram-level cGMP-compliant outputs for translational applications.
Purification Program
Multi-step chromatographic purification workflows (affinity, ion-exchange, size-exclusion) ensuring >95% purity across antibody classes.
Quality Testing and Analysis
Orthogonal characterization panels assessing structural integrity (SEC-HPLC, mass spec), functional activity (cell-based assays), and stability profiles (accelerated degradation studies) to meet regulatory compliance standards.
Case Study
The client provided the sequence of the target protein (named as P protein) with a length of 328 aa. Creative Biolabs was contracted to develop monoclonal antibodies targeting different epitopes on the P protein through mouse immunization and hybridoma screening. After hybridoma screening and identification, finally, we successfully developed 2 clones that can recognize different epitopes of the target protein with high affinity.
| Hybridoma screening | Hybridoma subclone supernatant gradient ELISA |
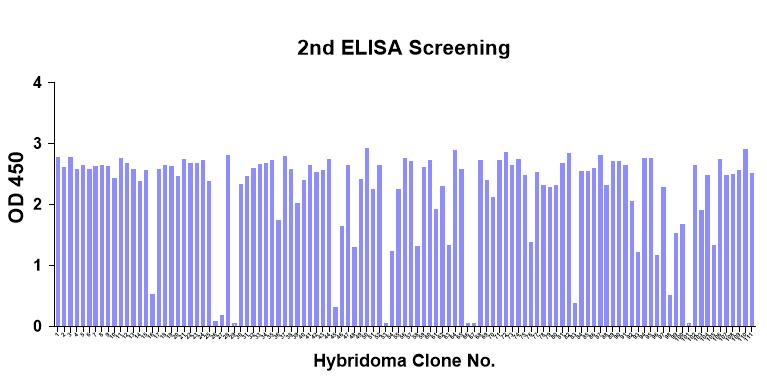
|
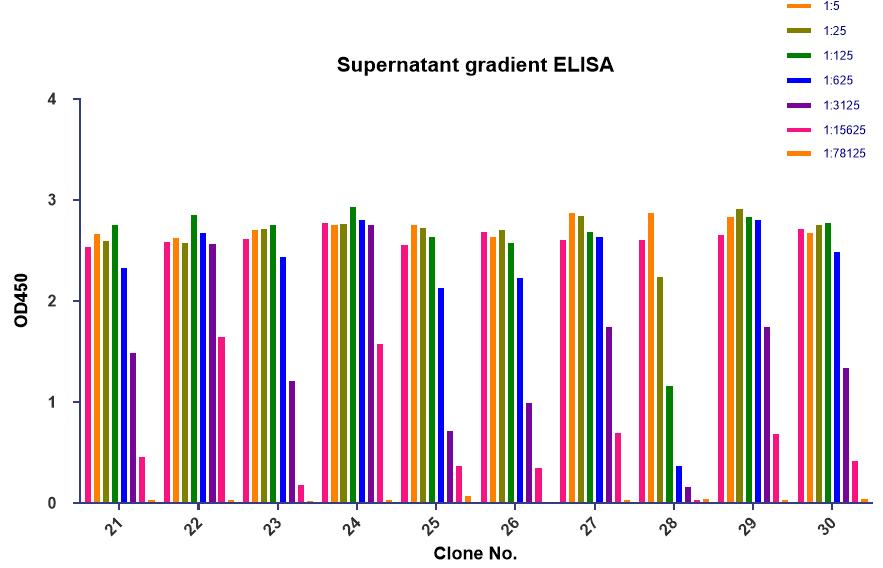
|
| Antibody Production | Sandwich ELISA |
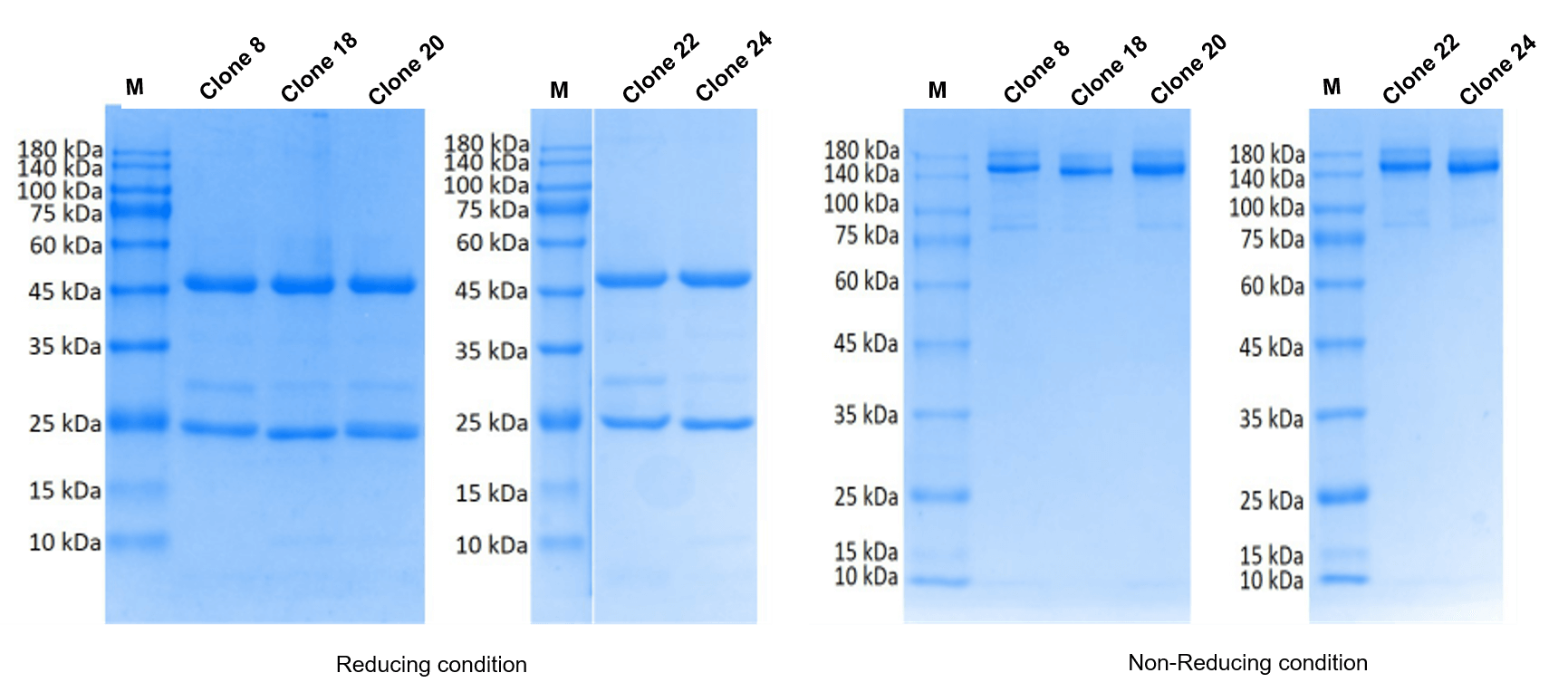
|
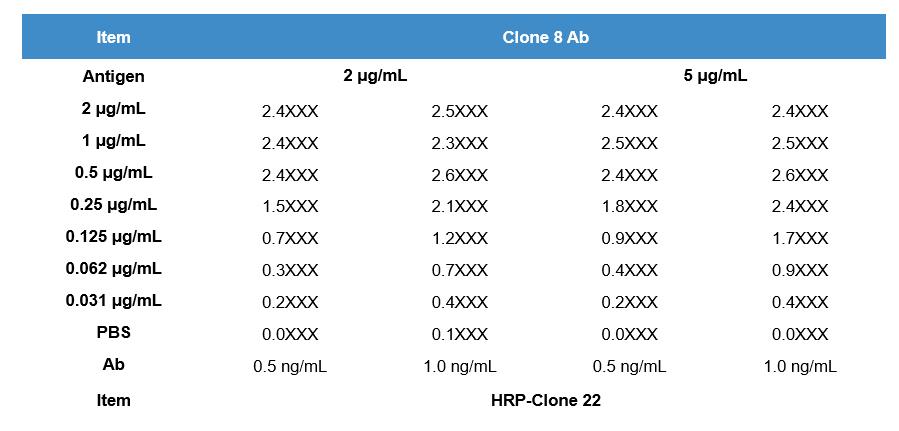
|
Applications of Monoclonal Antibody
Research
mAbs are pivotal in biological research for isolating specific molecular targets. Their precision enables protein quantification via ELISA, antigen detection in Western blots, and cellular marker analysis through flow cytometry. Immunohistochemistry applies mAbs to map protein localization in tissues, revealing structural and pathological insights. These methodologies support critical investigations into protein function, cellular behavior, and disease mechanisms.
Diagnostics
mAbs underpin modern diagnostic systems by detecting disease-specific biomarkers. Rapid tests leverage mAbs for pathogen identification in infections, while oncology diagnostics use tumor-targeting mAbs to stage malignancies. Quantitative immunoassays track biomarkers in bodily fluids, aiding treatment monitoring and disease progression assessment.
Therapeutics
mAbs enable targeted therapies across medical disciplines. In oncology, they directly neutralize cancer cells or deliver cytotoxic agents selectively. Autoimmune therapies deploy mAbs to block inflammatory pathways (e.g., TNF-α inhibition) or deplete pathogenic immune cells. Infectious disease management utilizes mAbs to neutralize pathogens, exemplified by SARS-CoV-2 treatments. Their specificity minimizes off-target effects, offering safer alternatives to conventional therapies while addressing diverse clinical challenges.
Published Data
1. Development of an anti-Pseudomonas aeruginosa Therapeutic Monoclonal Antibody
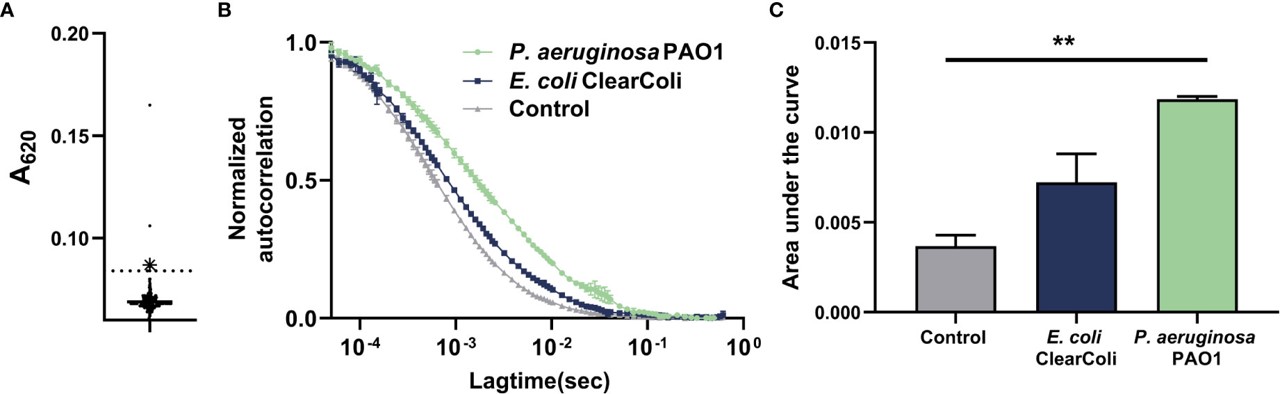 Fig.4 Generation of mAbs against P. aeruginosa.3,5
Fig.4 Generation of mAbs against P. aeruginosa.3,5
In this study, researchers developed a novel therapeutic mAb against P. aeruginosa using hybridoma technology. They characterized the antibody's binding affinity to various clinical strains through ELISA and fluorescence correlation spectroscopy. In vitro, the anti-P. aeruginosa antibody facilitated opsonophagocytic killing by murine macrophages and enhanced complement-mediated killing. Prophylactic treatment with anti-P. aeruginosa antibody in a mouse model of acute pneumonia resulted in improved clinical outcomes and a reduction in P. aeruginosa burden in the respiratory tract compared to untreated controls. This study offers promising pre-clinical data for anti-P. aeruginosa antibody's therapeutic potential against P. aeruginosa infections.
2. Development of Monoclonal Antibodies Against Oropouche Virus and its Applicability to Immunohistochemical Diagnosis
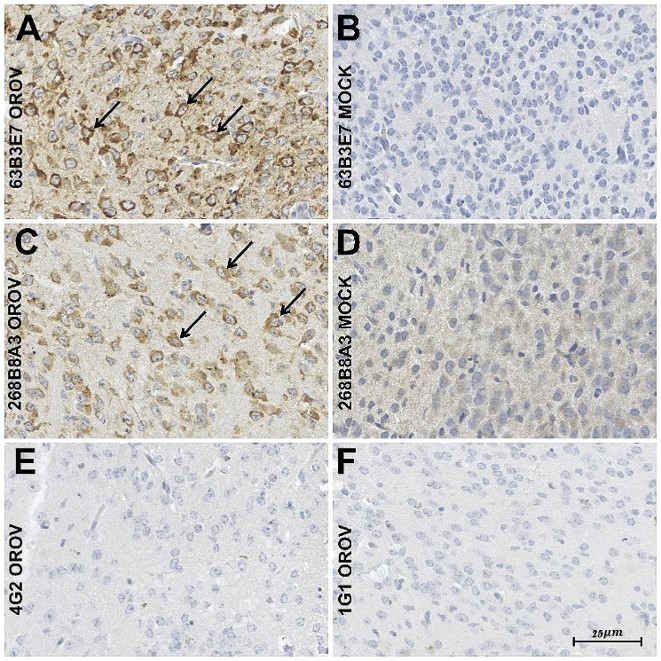 Fig.5 Applicability of mAbs in immunohistochemistry of OROV-infected mouse neuronal tissue.4,5
Fig.5 Applicability of mAbs in immunohistochemistry of OROV-infected mouse neuronal tissue.4,5
Due to the challenges in diagnosing Orthobunyavirus oropouche ense virus (OROV), the causative agent of Oropouche fever, this study detailed the development and characterization of mouse mAbs against a Brazilian strain of OROV. The study demonstrated the potential of these mAbs as diagnostic tools for detecting the OROV nucleocapsid in indirect immunofluorescence (IFA) and immunohistochemistry (IHC) assays. Beyond diagnostic applications, these mAbs could also be utilized for serological surveys and epidemiological surveillance to track the spread and prevalence of OROV.
Q&A
-
Q: What are the core advantages of your services?
A: We synergize technical mastery with collaborative engagement to advance antibody innovation. Core strengths include: 1) Expert Teams: Researchers skilled in immunology, protein engineering, and hybridoma development drive project execution. 2) Advanced Infrastructure: Labs equipped for hybridoma generation, high-throughput screening, and mammalian expression accelerate development phases. 3) Quality Validation: Binding kinetics, epitope characterization, and stability assessments ensure antibody reliability. 4) Close Customer Cooperation: Real-time communication channels and iterative strategy adjustments align deliverables with project objectives. Milestone tracking and joint problem-solving maintain alignment across experimental and regulatory demands.
-
Q: What antibody formats do you produce?
A: Our capabilities extend to multiple structural configurations, including full-length IgG/IgM isotypes and engineered fragments (Fab, scFv), developed to align with distinct experimental or therapeutic objectives.
-
Q: Is large-scale therapeutic antibody production feasible?
A: Our production infrastructure supports customizable scales, from pilot batches for preclinical validation to regulatory-compliant manufacturing for clinical trials and commercial distribution.
-
Q: How is product quality ensured?
A: Multi-phase quality assurance protocols integrate in-process analytical testing, structural/functional validation (binding affinity, purity assays), and batch-specific documentation to meet pharmacopeial standards across development phases.
-
Q: Is technical assistance available post-project completion?
A: Post-project technical consultations remain available, spanning antibody validation protocols, diagnostic assay optimization, and long-term antibody supply. Collaborative partnerships persist through iterative refinement processes to address application-specific challenges.
References
- Roth, Kristian Daniel Ralph, et al. "Developing recombinant antibodies by phage display against infectious diseases and toxins for diagnostics and therapy." Frontiers in Cellular and Infection Microbiology 11 (2021): 697876. Doi: 10.3389/fcimb.2021.697876.
- Yang, Zhengxin, et al. "Rapid production of monoclonal antibodies from single mouse B cells against FMDV." Animal Diseases 4.1 (2024): 28. Doi: 10.1186/s44149-024-00133-y
- Horspool, Alexander M., et al. "Development of an anti-Pseudomonas aeruginosa therapeutic monoclonal antibody WVDC-5244." Frontiers in Cellular and Infection Microbiology 13 (2023): 1117844. Doi: 10.3389/fcimb.2023.1117844.
- Andreolla, Ana Paula, et al. "Development of monoclonal antibodies against oropouche virus and its applicability to immunohistochemical diagnosis." Virology Journal 21.1 (2024): 81. Doi: 10.1186/s12985-024-02323-z.
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.