Our kits enable precise investigation of protein expression levels, analysis of post-translational modifications like phosphorylation, and studying protein-protein interactions through techniques such as co-immunoprecipitation, providing deep insights into cellular processes and disease mechanisms.
Creative Biolabs stands at the forefront of in vitro diagnostics (IVD), specializing in the development of advanced Western blot-based kits. With extensive experience in IVD production, we deliver a comprehensive range of diagnostic solutions, particularly western blots, meticulously tailored for critical medical studies and clinical trials. Our commitment ensures unparalleled precision and reliability in disease diagnosis, infection detection, and biomarker analysis.
Western Blot
Western blot (WB), also known as immunoblotting, is a fundamental molecular biology technique employed for the definitive identification and quantification of specific proteins within complex biological samples. The process initiates with the separation of proteins by size using gel electrophoresis. Subsequently, these separated proteins are efficiently transferred onto a solid membrane, typically nitrocellulose or PVDF. The membrane is then incubated with highly specific primary antibodies that bind to the target protein, followed by enzyme-conjugated secondary antibodies for signal amplification and visualization. This multi-step approach provides robust qualitative and semi-quantitative data on protein presence, expression levels, and post-translational modifications.
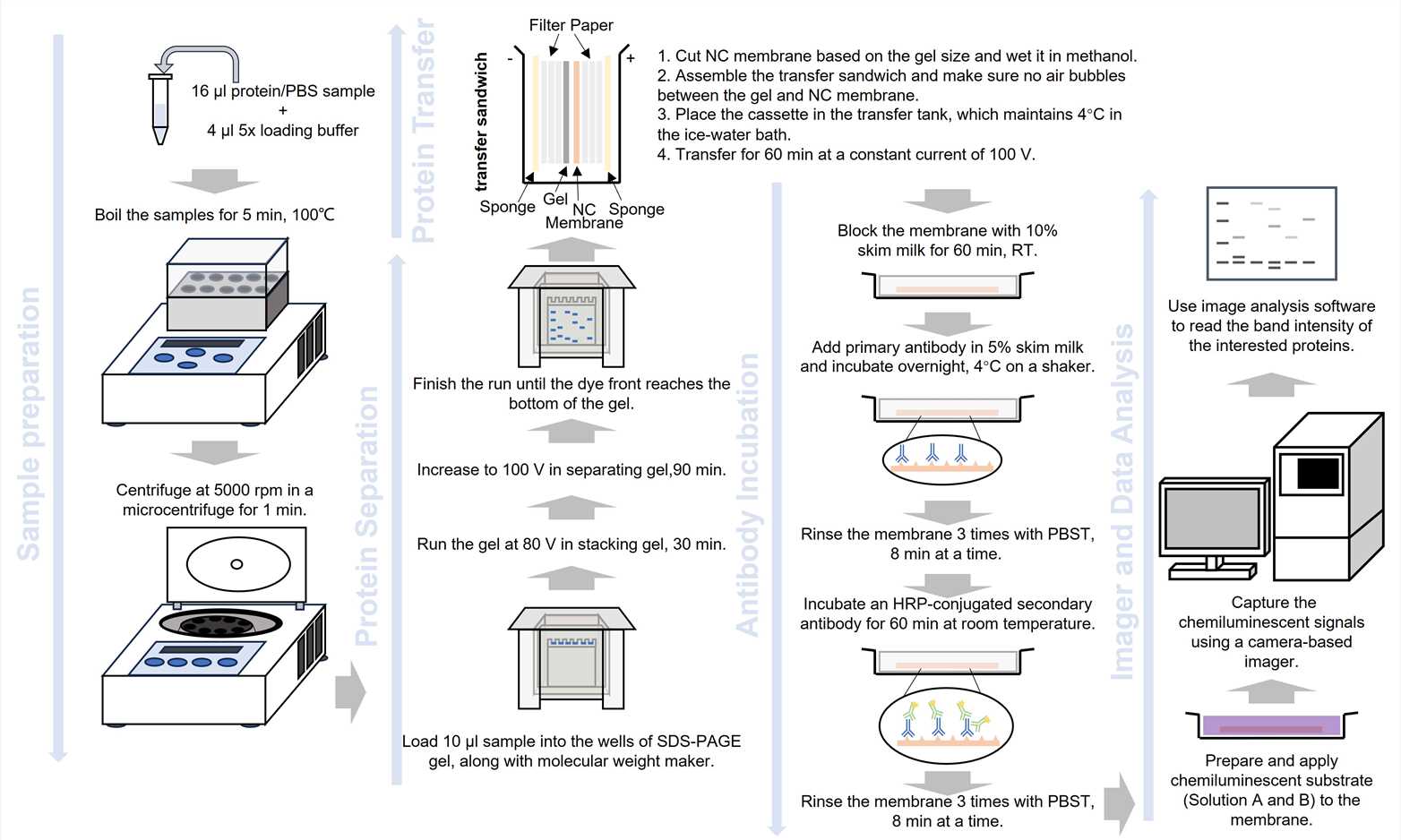 Fig.1 The workflow of WB.
Fig.1 The workflow of WB.
Advantages of Our WB Platform
- High Specificity: The technique relies on highly specific antibody-antigen interactions, enabling precise detection of a target protein amidst a complex mixture, minimizing false positives.
- Protein Characterization: It provides information on protein size, allowing for the differentiation of isoforms, cleaved products, or post-translational modifications.
- Quantitative Analysis: With proper controls and optimized conditions, WB can be used for semi-quantitative or quantitative assessment of protein expression levels.
- Versatility: Adaptable to a wide range of sample types, including cell lysates, tissue homogenates, and biological fluids, making it broadly applicable across research and diagnostic fields.
- Validation Standard: Often considered a gold standard for validating protein expression data obtained from other less specific techniques.
Our WB Based Kits Development Services
At Creative Biolabs, we offer a specialized custom WB-based kit development service designed to meet the unique and exacting requirements of your research and diagnostic projects. Our service encompasses the comprehensive design, optimization, and manufacturing of complete WB kits, tailored to your specific protein targets and application needs. We leverage our deep expertise in antibody validation, reagent formulation, and quality control to deliver kits that promise superior performance, enhanced reproducibility, and streamlined workflows, empowering your team to achieve precise and reliable results with confidence.
Service Workflow of WB Based Kits Development
Our WB-based kit development projects follow a structured and transparent workflow, ensuring efficient progress and high-quality outcomes.
Our process starts with an initial consultation to define your specific target protein, intended application (e.g., diagnostic, research), desired kit components (e.g., gels, membranes, detection reagents), and critical performance criteria (e.g., sensitivity, specificity). This ensures clear project scope and objectives.
We identify and select optimal primary and secondary antibodies for your target. Rigorous antibody validation follows, including specificity testing with controls, affinity characterization, and cross-reactivity screening. This ensures optimal binding and minimal off-target interactions within the WB.
Our experts then conduct reagent formulation and optimization. This involves fine-tuning all necessary buffers (lysis, transfer, wash), blocking solutions (milk, BSA), and detection substrates (chemiluminescent, fluorescent) to maximize signal-to-noise ratios and ensure robust WB performance.
After reagent optimization, we proceed with component integration and assembly. This combines validated antibodies, optimized buffers, membranes, and consumables into a cohesive kit. We ensure compatibility and stability, designing user-friendly packaging and clear instructions for seamless WB execution.
Finally, comprehensive quality control and validation are performed on assembled kits. This involves running multiple WB with biological samples, positive/negative controls, and loading controls. We verify kit performance, reproducibility, and adherence to detection limits. Successful validation leads to the final delivery of your functional, high-quality WB kit.
Applications
Biomedical Research
Clinical Diagnostics
These kits are vital for detecting specific antibodies or antigens for infectious diseases (e.g., HIV, Lyme disease, Hepatitis), identifying cancer biomarkers, monitoring therapeutic responses, and profiling proteins for neurological disorder diagnosis.
Drug Discovery and Development
We provide essential tools for validating drug targets, assessing their expression, evaluating the efficacy of drug candidates by monitoring protein modulation, and screening for potential off-target effects of novel compounds.
Proteomics and Cell Biology
Our solutions facilitate confirming protein presence and purity in purification schemes, analyzing protein localization within cellular compartments, and comprehensively studying cellular signaling pathways and protein turnover dynamics.
Published Data
1. Development of Western Blot for Detecting Antibodies Against B. abortus
Tab.1 A comparison of results from RBT, CFT, and WB for animals in group II.1,3
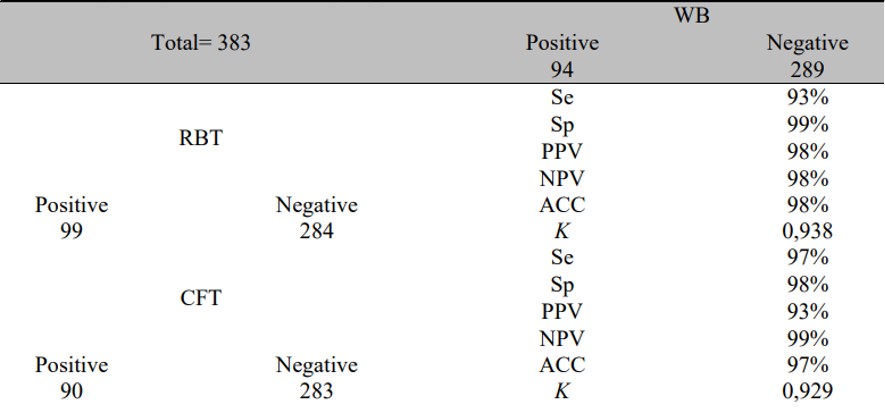
This study developed and standardized a WB test to detect antibodies against Brucella abortus in cattle. Serum samples were collected from two groups: Group I comprised 60 serum samples from vaccinated animals, including 30 RBT-positive infected samples and 30 RBT-negative samples (true positive and true negative); Group II included 383 field samples, with 90 positive and 293 CFT-negative sera. The WB identified a key band at ≤ 20 kDa, effectively distinguishing infected from non-infected cattle. Compared to the Rose Bengal Test (RBT), the WB showed a sensitivity of 93%, specificity of 99%, and accuracy of 98%, with a kappa value of 0.938. When compared to the Complement Fixation Test (CFT), sensitivity was 97%, specificity 98%, and accuracy 97%, with a kappa of 0.929. This WB test is a serological test and offers potential as a confirmatory tool for diagnosing bovine brucellosis.
2. Validation of Western Blot for Histoplasma capsulatum Antibody Detection.
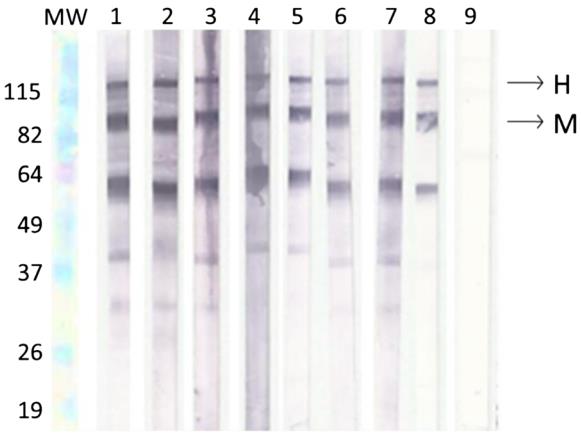 Fig.3 Analysis of the viability of membranes.2,3
Fig.3 Analysis of the viability of membranes.2,3
This study aimed to validate a WB test for detecting antibodies in histoplasmosis diagnosis. Researchers used 118 serum samples from histoplasmosis patients and 118 control samples to conduct this study. They also evaluated the viability of deglycosylated histoplasmin antigen (ptHMIN) on nitrocellulose membranes. The WB using ptHMIN showed high sensitivity, specificity, and faster results compared to traditional tests. This method is a valuable diagnostic tool for histoplasmosis, suitable for public health settings with limited laboratory resources. Additionally, the WB test offers rapid results and exhibits almost perfect reproducibility, making it a reliable option for microbiology laboratories.
Service Highlights
- Rigorous Antibody Validation: We employ multi-pillar validation strategies, including genetic and orthogonal methods, guaranteeing exceptional antibody specificity and minimal off-target binding.
- Optimized Reagent Formulation: Every buffer, blocking agent, and detection substrate is meticulously optimized for superior signal-to-noise ratios and consistent performance across diverse sample types.
- Comprehensive Quality Control: Our kits undergo extensive internal validation with positive, negative, and loading controls, ensuring reliability and reproducibility for every batch.
- Customization and Flexibility: We offer tailored kit configurations to precisely match your unique research needs, from specific protein targets to preferred detection methods.
- Expert Scientific Support: Our team of experienced biologists provides dedicated technical assistance and troubleshooting guidance throughout your experimental journey.
FAQs
-
Q: Can you develop WB kits for detecting low-abundance proteins?
A: We can indeed develop WB kits optimized for low-abundance proteins. This is achieved through careful selection of high-affinity antibodies, incorporation of highly sensitive detection substrates, and optimization of signal amplification protocols to maximize detection limits.
-
Q: What types of detection methods are supported by your WB kits?
A: Our WB kits support various detection methods. We primarily focus on highly sensitive chemiluminescent and fluorescent detection systems, which offer robust signals, wide dynamic ranges, and suitability for quantitative analysis, depending on your specific application needs.
-
Q: How do you address issues of sample variability and degradation in kit design?
A: We address sample variability and degradation by providing clear guidelines within our kit protocols for proper sample collection, handling, and storage. While sample preparation is user-dependent, our kits are designed to be robust against minor variations and include controls to monitor sample integrity.
-
Q: Do your WB kits include all necessary controls for experimentation?
A: Our WB kits are designed to include all necessary controls for robust experimentation. This typically comprises positive control lysates, negative control lysates, and loading controls, which are crucial for validating assay performance and ensuring accurate data interpretation.
-
Q: Are your WB kits compatible with different types of blotting membranes?
A: Our WB kits are developed with compatibility in mind. Our protocols are optimized for commonly used blotting membranes, such as PVDF and nitrocellulose, and we can provide guidance on membrane selection to ensure optimal protein transfer and binding for your specific application.
-
Q: Can your kits be specifically optimized for quantitative WB analysis?
A: Our kits can be specifically optimized for quantitative WB analysis. This optimization includes ensuring linearity of signal across a broad protein concentration range, minimizing background noise, and providing robust loading controls to facilitate precise and accurate protein quantification.
-
Q: Do you offer post-development support or troubleshooting for its WB kits?
A: We offer comprehensive post-development support and troubleshooting for its WB kits. Our team of expert scientists is readily available to provide technical assistance, answer your questions, and help resolve any challenges you may encounter during your experiments.
With decades of specialized experience and a steadfast commitment to scientific excellence, Creative Biolabs stands as your ideal partner for WB-based kit development. We invite you to contact us to discuss your specific project needs and discover how our tailored solutions can accelerate your research and diagnostic endeavors.
References
- Falcão, Marcus Vinícius Dias, et al. "Development and standardization of a western blotting test for detection of antibodies against B. abortus." Arquivo Brasileiro de Medicina Veterinária e Zootecnia 71 (2019): 160-166.
- Almeida, Marcos de Abreu, et al. "Validation of western blot for Histoplasma capsulatum antibody detection assay." BMC Infectious Diseases 16 (2016): 1-8.
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.