Acute Toxicity
Animal toxicity is a very important step in IND-enabling in drug discovery and development towards medical use. The first important aspect to consider in animal toxicity is the acute toxicity. Acute toxicity is referred to as the unwanted effects that occur on the research subject immediately or soon after the administration (single or multiple) of the drug candidate within 24 hours. Any changes or effects in the tested object that may lead to biochemical lesions, functional impairments in organs, and interference in the function of individual or multiple organs can be referred to as unwanted effects. To determine dose range for safe administration, the dosage that doesn't cause any adverse effect and the dosage that is life-threatening are measured. Creative Biolabs provides multiple platforms and approaches for acute toxicity evaluation for drug candidates.
In an acute toxicity test, the test compound should be given to the test objects in the same way that it is intended to be taken by humans. As the routes of administration affect the amount of the compound that reaches the blood stream and its biodistribution, using the same route in the toxicity test can best reflect the in vivo situation. The test objects are then observed after the administration for all kinds of toxic effects. Such studies can provide not only safety information but also general data such as the dose-response relationship. After 2 weeks, test objects (that survived) are killed, dissected, and analyzed for any further signs of toxicity. Acute toxicity test results provide information about the proper dosage for clinical applications as well as what kinds of/how/when toxicity may occur. Further studies can be planned accordingly. Since some degree of toxicity is normally inevitable and allowed in pharmacy, especially for drugs dealing with life-threatening diseases, compounds showed toxicity in acute toxicity tests are not necessarily abandoned. Most of the time, by adjusting the dosage and method or times of administration, toxicity can be maintained under an allowable level.
Regularly, a dose-dependent unwanted effect(s) measurement is established in an acute toxicity evaluation. Lethal dose (LD50), which is the dose given all at once that kills 50% of test objects, is currently used as a major parameter of acute toxicity. LC50 value (LC stands for lethal concentration) which is the concentration of the chemical in air at which 50% of the test animals are killed during the observation period, is also a useful parameter. Other commonly used terms include LD01 (lethal dose for 1% of the test population, also indicates the dosage that causes low-to-no adverse effects), LD100 (lethal dose for 100% killing), LDLO (the lowest dose that causes death), and TDLO (the lowest dose that causes a toxic effect). Besides mortality, other parameters such as other unwanted biological effects, time of onset, duration of onset, and degree of recovery of survived animals, are also important in the evaluation.
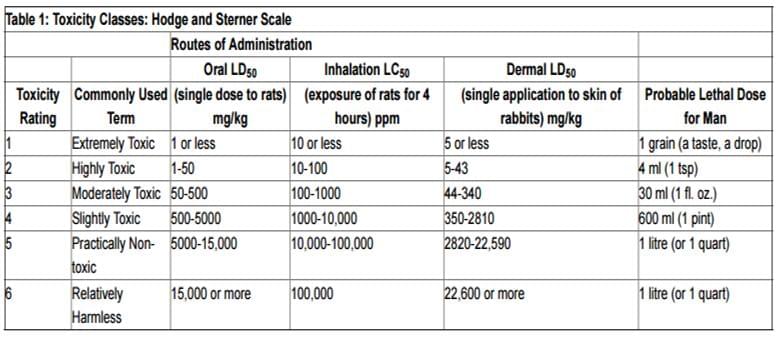 Table1. An example table of toxicity rating (www.ccohs.ca)
Table1. An example table of toxicity rating (www.ccohs.ca)
Creative Biolabs is dedicated to providing customers one-stop service from target identification to IND-enabling tests such as acute toxicity measurement. We developed multiple platforms for preclinical tests of drug candidates. For more detailed information, please feel free to contact us or directly sent us an inquiry.
For Research Use Only.
