ADA Assay Kit Development
Immunogenicity test is a critical component of biotechnology-derived biopharmaceutical drug development as anti-drug antibodies (ADA) can impact drug exposure by affecting the ability of the drug to reach the intended target, alter the PK profile, and even mediate serious adverse effects. As a leading service provider in the area of immunogenicity assessment, Creative Biolabs offers high-quality ADA assay kits development services to help worldwide customers measure ADA in biological matrices.
Kits Development for ADA Detection
Traditionally, ELISAs and Electrochemiluminescence (ECL) have been widely used in the identification of the presence of ADA. Though effective for detection, ELISA methods often fail to adequately measure specific antibody response in the presence of circulating protein therapeutic due to the limitation on sensitivity and problems presented on a plate-based format. However, FDA Guidance (2019) has stated that ADA assays should be sufficiently sensitive to detect low levels of ADA before the amount of ADA impact the PK, PD, safety, or efficacy. Therefore, new methods and kits should be developed for ADA detection. Based on the high-end technology platform for ADA assay kits development, Creative Biolabs can deliver multiple types of high-affinity custom ADA assay kits for global researchers, which including but not limited to:
-
Anti-Drug Antibody ELISA Kits
Anti-drug antibodies (ADA) are unwanted antibodies produced by the body's immune system in response to the therapeutic antibody administrated. This will have the potential to affect product pharmacokinetics, pharmacodynamics, safety, and even result in adverse side effects including the autoimmune response. Detection and monitoring the level of ADA is important to fully understand the potential immune responses.
-
Anti-ID Antibody ELISA Kits
Anti-idiotype antibodies are antibodies that bind to the variable region of another antibody. They have been widely used in vaccine development, antibody drug immunogenicity, and pharmacokinetic studies. ELISA testing is most widely used in the confirmation of the specificity and functionality of anti-idiotype antibodies.
-
Biosimilars Antibodies ELISA Kits
Biosimilar antibodies are antibodies almost identical to their therapeutic counterpart, serving the same therapeutic function. The development of biosimilar antibodies will encounter a variety of challenges, among which immunogenicity will prove to be the biggest one. Proper design and validation of a kit to detect biosimilar antibodies will prove integral to developing a biosimilar protein.
-
Therapeutic Antibodies ELISA Kits
Therapeutic antibodies have been widely used for the treatment of numerous diseases, such as cancer and autoimmune disease. In addition to targeting specific proteins on certain cells to invoke an immune response against that protein or cell type, these antibodies can also target and attack unwanted cell types in the body. ELISA kits are designed to detect free therapeutic antibodies in serum & plasma samples.
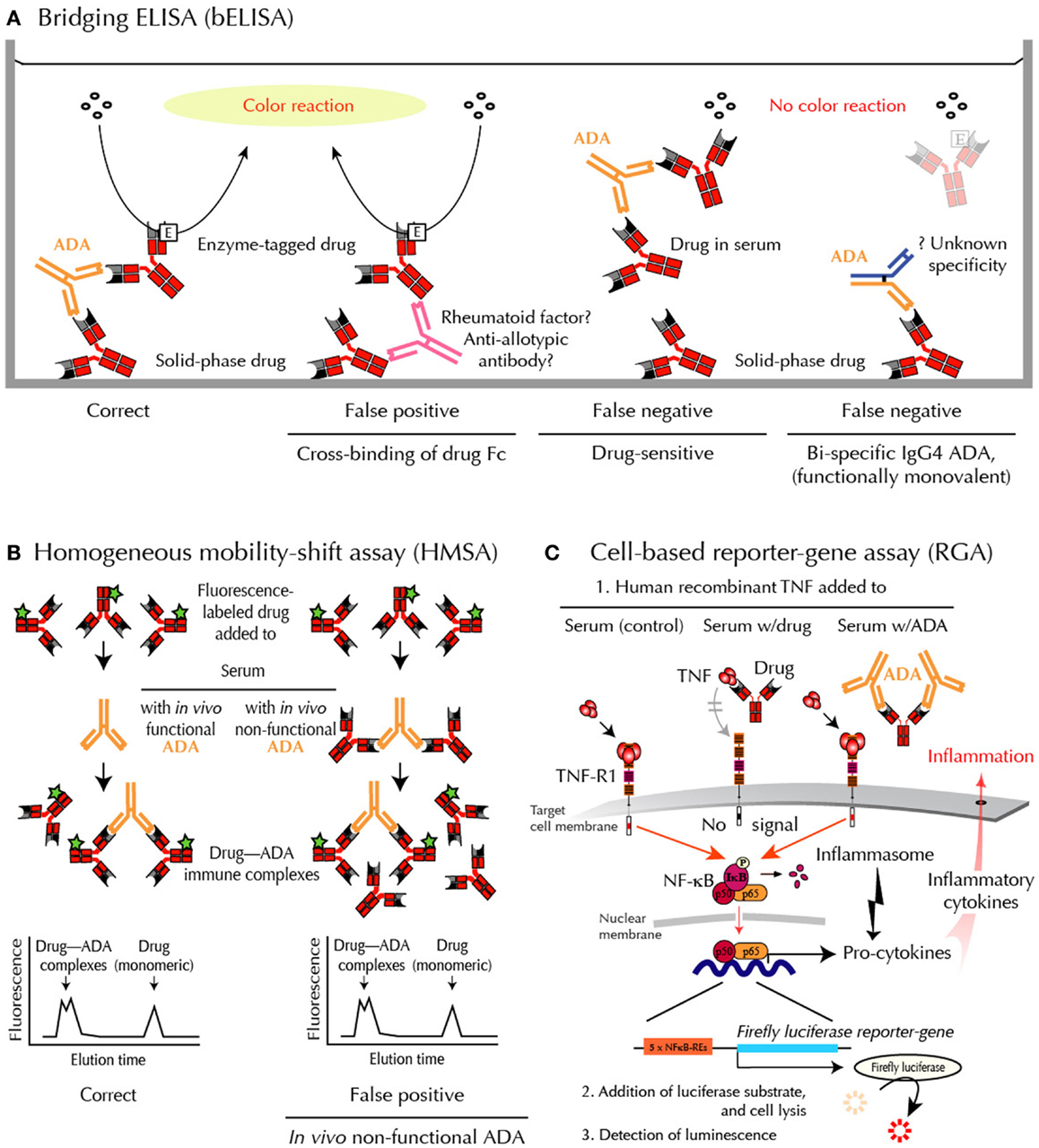 Fig.1 Methods for ADA detection. (Bendtzen, 2015)
Fig.1 Methods for ADA detection. (Bendtzen, 2015)
We are committed to offering high-quality ADA assay kits for the investigation of the mechanism of the immune response. Our assays are designed to minimize the effect of different biological matrices on the performance of the assay while maintaining appropriate levels of sensitivity. We promise all kits we developed are easily adaptable to meet global regulatory guidelines on bioanalytical method validation.
| Development protocol | Explanation |
| Antigen | Provided by sponsors |
| Antibody production and purification |
Polyclonal/Monoclonal Antibody Production Antibody purification |
| Select of ELISA types | Bridge Format, Indirect protein A/G Format, Indirect IgG/M/E format, Competition ELISA |
| Optimization & Validation | Linearity, Accuracy, Precision, Sensitivity, Cross-reactivity test |
| QC test | |
| Mass production of kit |
Highlights
- Both standard and customized protocols to satisfy each client's requirements
- Multiple assay types available for option
- Validated kits with excellent sensitivity and antibody capture rate
- Simple operation steps
With extensive experience and expertise in developing immunoassays for the assessment of immunogenicity, Creative Biolabs is dedicated to helping global scientists create high-affinity custom ADA assay kits in compliance with global regulatory guidelines on bioanalytical method validation. Besides ADA assay kits development, we also provide anti-drug antibodies development services. For more detailed information, please feel free to contact us.
Reference
- Bendtzen, K., (2015). Immunogenicity of anti-TNF-α biotherapies: II. Clinical relevance of methods used for anti-drug antibody detection. Frontiers in immunology, 6, p.109.
For Research Use Only.
