Anti-Drug Antibody Development
Anti-drug antibodies (ADA) are critical components for the development of preclinical PK/PD and immunogenicity assays of multiple biotechnology-derived biopharmaceutical drugs. When developing a kit or assay, the most important step is developing antibodies with high affinity and specificity to the target antibody. Supported by our advanced technology platform and experienced scientists, Creative Biolabs offers customized ADA antibody development services to accelerate your programs and meet your study milestones.
Introduction of ADA Antibody
An anti-drug antibody refers to an antibody that can recognize and bind to the idiotype of another antibody, generally an antibody drug. In the past years, ADA antibodies have been extensively used in the detection and quantification of therapeutic antibodies during the drug development process, including pharmacokinetic and immunogenicity assays. Generally, pharmacokinetic parameters of monoclonal antibody drugs are quantified by ligand binding assays. Compared with ligand binding assay, ADA antibodies provide a more accurate quantification method which is of great importance for the assessment of the exposure-response relationship to determine the optimal dose and safety parameters. Except for pharmacokinetic study, ADA antibodies have also been used as a reference for immunogenicity assays owing to their ability to bind specifically to antibody drugs.
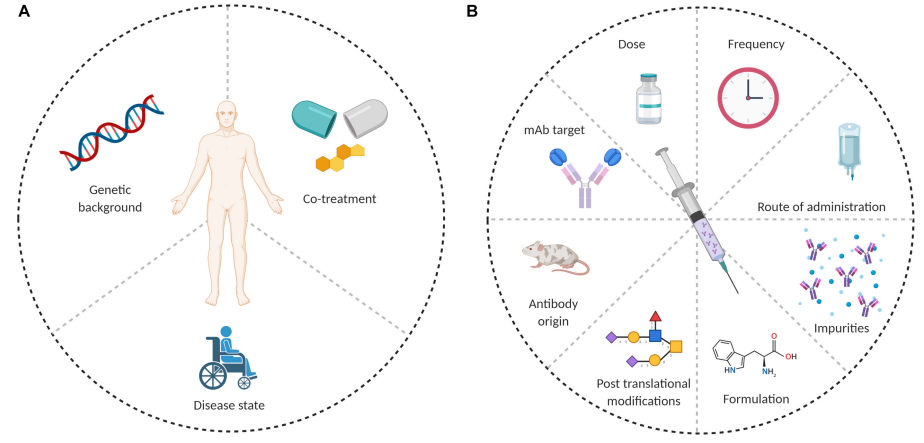 Fig.1 Possible causes of ADA formation.1, 2
Fig.1 Possible causes of ADA formation.1, 2
-
Anti-ID Antibodies for PK Assay
The ability to specifically detect and measure the antibody drug from serum samples is imperative for the ability to develop a sensitive PK immunoassay. Anti-idiotype antibodies are most commonly used for pharmacokinetic (PK) assays as they are capable of binding to antibody drugs within biological fluids. Currently, various anti-idiotype antibody based methods have been developed by Creative Biolabs for preclinical PK study.
-
Anti-ID Antibodies for ADA Assay
Anti-Drug Assays (ADA) are typically used to measure the immunogenicity of the antibody drug. These assays are typically in the format of a bridging assay and use the patient sera as the "bridging antibody". Positive controls for this assay are necessary and therefore anti-idiotype antibodies are generated as a reference.
-
Anti-ID Antibodies for Neutralizing Assay
Characterization of anti-drug antibodies (ADAs) for neutralizing activity is a crucial component of safety and efficacy evaluations in the clinical development of biopharmaceuticals. Anti-Id antibodies are often used as controls for antibody neutralization assays and blocking assay for the ability to neutralize or block specific ligand binding of a therapeutic antibody.
-
Anti-ADC Antibodies
ADCs are complex biotherapeutics that consist of a monoclonal antibody (mAb) covalently linked to a cytotoxic agent through a stable linker. ADCs can theoretically pose a risk of immune responses in patients as ADAs can develop against the different domains in the ADC such as mAb epitopes, mAb neoepitopes, the linker, and the cytotoxic agent. Anti-ADC antibodies are developed and used to assess the immunogenicity of ADCs.
Anti-Drug Antibodies Development Services at Creative Biolabs
With years of experience in the field of antibody development and engineering, Creative Biolabs offers a full package of anti-drug antibody development services to support your study. Our scientists are professionals in developing high specificity and affinity antibodies from various species (including rabbit, chicken, llama, camel, alpaca, cow, dog, mouse, rat, sheep, and human). Moreover, we have successfully established multiple antibody development platforms, covering hybridoma, phage display, B cell sorting…
Highlights
- High-throughput antibody expression and screening technology
- Flexible protocols with a fast turnaround time
- High specificity and binding affinity
- Best after-sale services with more than 98% success rate
Creative Biolabs is committed to accelerating the development of global customers' monoclonal antibody drugs. With advanced technology platforms and professionals, we are capable of providing custom anti-drug antibody production services and products to meet our customers' needs. For more detailed information, please feel free to contact us or directly send us a quote.
References
- Vaisman-Mentesh, Anna, et al. "The molecular mechanisms that underlie the immune biology of anti-drug antibody formation following treatment with monoclonal antibodies." Frontiers in immunology 11 (2020): 1951.
- Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
