Anti-Drug Antibody (ADA) Services
As a pioneer company in the field of biological industry, Creative Biolabs has gained rich experience which enables us to provide a series of high-quality anti-drug antibody services for potential immunogenicity evaluation. The services we provide will strictly be in compliance with the current FDA and EMEA guidelines as well as AAPS white papers.
Introduction of ADA
During the past years, biological agent usage has rapidly increased. However, many factors still limit their usage. Studies have shown that almost all protein-based biotherapeutics have the potential to induce the production of anti-drug antibodies (ADAs), which may lead to a loss of efficacy and/or increasing the risk of adverse reactions (e.g. infusion reactions, anaphylaxis, or immune-complex-mediated diseases). In particular, the ADA response is directed not only to the administrated biotherapeutic but also to its endogenous counterpart protein and may elicit a life-threatening response if the endogenous protein is unique and nonredundant and has a vital life function. To avoid the unwanted outcomes, the regulatory agencies request that a biotherapeutic's ADA ability be assessed and a determination of its characteristics relative to any induced clinical consequences be done as part of the approval process for biotherapeutics.
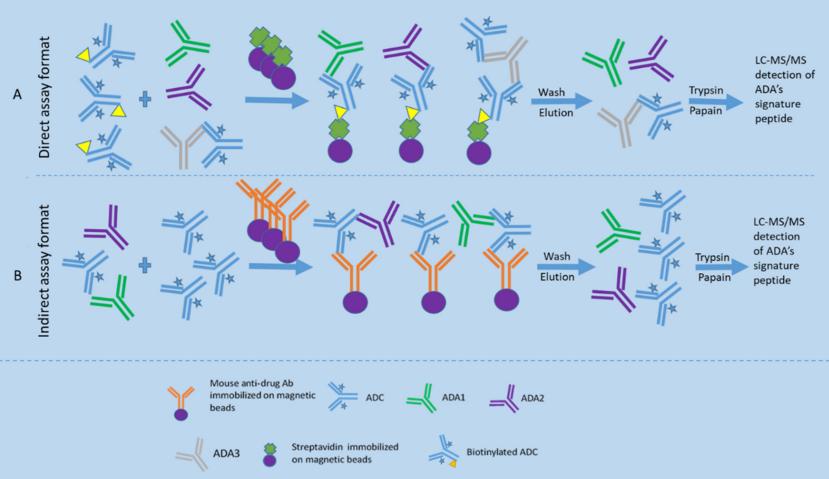 Fig.1 Direct and indirect LBA-LC-MS/MS assay formats for ADA detection.1, 2
Fig.1 Direct and indirect LBA-LC-MS/MS assay formats for ADA detection.1, 2
Anti-Drug Antibody (ADA) Services Available at Creative Biolabs
ADA can be used as a positive control or reference standard in immunogenicity assays. Therefore, numerous functional antibodies for ADA development are required. With a wealth of experience, Creative Biolabs offers multiple robust technologies for worldwide customers, including hybridoma technology, phage display technology, and single B cell sorting technology. Both polyclonal and monoclonal antibodies against desired targets can be generated. We have the most commonly applied animals for antibody production in immunogenicity assessment - rodents and monkey. The ADA we offer can be presented in different formats (Fab, scFv, VHH) with high-affinity to meet your specific requirements.
Both preclinical and clinical studies have convinced that ADA could lead to significant changes in toxicology, pharmacokinetics, and efficacy. To evaluate the immunogenicity of ADAs comprehensively, a multi-step analytical assay including a broad range of different assay formats and methodologies should be conducted. We are experts at assay development and confirmation. Based on our cutting-edge technology, Creative Biolabs is able to offer a series of antibody immunogenicity assessment services for worldwide customers.
It is critical to assess the immunogenicity risk of potential biotherapeutics in producing neutralizing and non-neutralizing anti-drug antibodies, especially in clinical phases of drug development. During the past years, we have successfully developed different assay methodologies for ADA detecting, including ELISA, radioimmunoassay, surface plasmon resonance, and electrochemiluminescence-based technologies. In particular, multiple labeling approaches have been used for immunogenicity evaluation.
Immunogenicity test is a critical component of biotechnology-derived biopharmaceutical drug development as anti-drug antibodies (ADA) can impact drug exposure by affecting the ability of the drug to reach the intended target, alter the PK profile, and even mediate serious adverse effects. As a leading service provider in the area of immunogenicity assessment, Creative Biolabs offers high-quality ADA assay kits development services to help worldwide customers measure ADA in biological matrices.
Anti-drug antibodies (ADA) are critical components for the development of preclinical PK/PD and immunogenicity assays of multiple biotechnology-derived biopharmaceutical drugs. When developing a kit or assay, the most important step is developing antibodies with high affinity and specificity to the target antibody. Supported by our advanced technology platform and experienced scientists, Creative Biolabs offers customized ADA antibody development services to accelerate your programs and meet your study milestones.
Features of our Services
- High quality with affordable price
- Professional technical team with abundant experience
- Both standard and customized protocols
- Best after-sale service
Aided by advanced technology and experienced scientists, Creative Biolabs is able to help you choose the optimal analytical method to yield high-quality data results. Specifically, our scientists are experienced in supporting immunogenicity studies in a variety of species including rodents, humans, and non-human primates. In addition to the services, we have many ready to use ADA products. To learn more detailed information, please feel free to contact us.
References
- Qin, Qiuping, and Likun Gong. "Current Analytical Strategies for Antibody–Drug Conjugates in Biomatrices." Molecules 27.19 (2022): 6299.
- Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
