Antibody Characterization & Analysis
Monoclonal antibodies (mAbs) have emerged as revolutionary therapeutic agents for an array of human diseases. As a leading company in the field of antibody development, Creative Biolabs offers a wide range of antibody characterization and analysis services to promote global customers’ antibody development programs. Our professional scientists are competent in performing such analysis to make sure your project a success.
Monoclonal antibodies (mAbs) have proven to be effective tools for use by both the pharmaceutical and diagnostic assay industries. To date, numerous methods have been developed for antibody production. However, these molecules are susceptible to enzymatic and chemical modification, aggregation, and degradation throughout the development, manufacture, and storage processes, which can negatively affect the pharmacokinetic properties, performance, and biological activity of the antibody products. Thus, it is of great importance to characterize and monitor antibodies for these types of changes, as well as to understand their effect on stability and function.
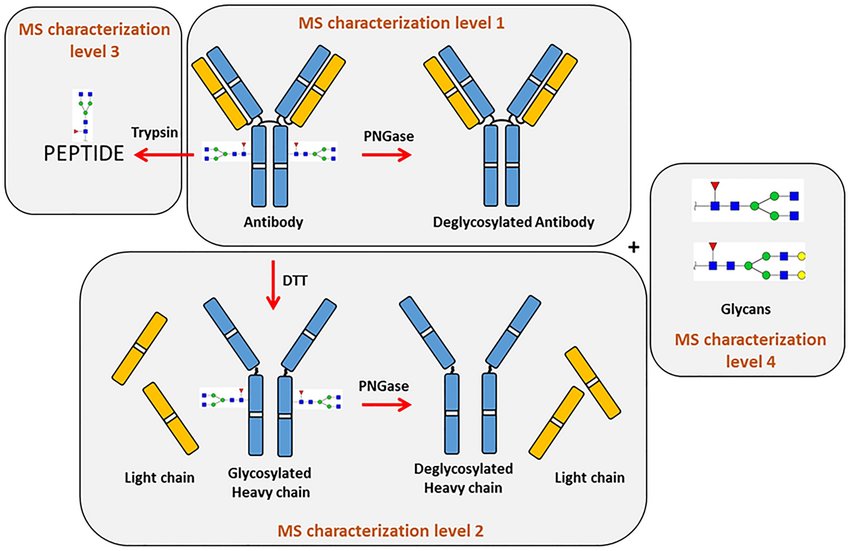 Fig. 1 Schematic representation of the workflow used for mAbs analysis, highlighting the four characterization levels explored.1
Fig. 1 Schematic representation of the workflow used for mAbs analysis, highlighting the four characterization levels explored.1
With years of experience, Creative Biolabs has established advanced cheminformatics- and biophysical-based analytical platforms with high sensitivity and resolution. These analytics can be tailored according to your specific needs with a focus on monitoring relevant critical quality attributes (CQAs), demonstration that process changes do not impact physicochemical properties and structure, the presence of product-related impurities or process-related impurities. We also offer support analytics to help you to demonstrate consistency or comparability of manufactured batches or as release tests for clinical trial materials or on-going GMP batch release tests. Moreover, we offer a range of in vitro antibody function assays to help customers further understand the structure/function relationships.
Services at Creative Biolabs
- Antibody Structure Analysis
- Post-Translational Modifications (PTMs) Analysis
- Antibody Aggregation Analysis
- Antibody Homogeneity Analysis
- Antibody Stability Analysis
- Analytical Comparability Studies of mAb Therapeutics
- Antibody Forced Degradation Studies
Highlight Features
- Extensive experiences in antibody development and characterization
- Interdisciplinary efforts across scientists from various fields
- Advanced one-stop drug discovery platforms
- Experienced multidisciplinary team with best after-sale service
Creative Biolabs is committed to accelerating your mAb product development and manufacture. Our advanced analytical techniques and years of experience allow us to deliver data with the highest sensitivity, accuracy, and resolution. In addition to mAbs, our expertise also spans related products such as biosimilars, Fc-fusion proteins, Fab fragments, and Fc fragments. For more detailed information, please feel free to contact us.
Reference
- Gomes, R.A., et al. Exploring the analytical power of the QTOF MS platform to assess monoclonal antibodies quality attributes. PloS one. 2019, 14(7). Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
