Antibody Host Cell Protein (HCP) Analysis
Recombinant proteins and other biopharmaceuticals (including monoclonal antibodies (mAbs)) are produced by living organisms, from which impurities can be derived, including host cell components, compounds of the culture media, and product variants and isoforms, heading this list are host cell proteins (HCPs). As a leading CRO company with years of experience and a comprehensive spectrum of validated analytical methods, Creative Biolabs is able to offer professional HCP analysis services using appropriate orthogonal methods.
Introduction to Host Cell Proteins (HCPs)
HCPs are a heterogeneous, complex group of proteins characteristic of the host cell. During the purification process, the majority of the HCPs can be removed but small amounts remain in the products, which can potentially affect drug efficacy and cause immunogenicity. Anti-HCP antibodies have been reported to cause adverse events by inducing immune-mediated clinical sequelae such as injection site reactions, flu-like symptoms, and at worst case, anaphylaxis. As a result, detection and quantification of residual HCPs are critical for biopharmaceutical companies in accordance with regulatory agency guidelines (e.g., ICH Q6B), requiring that biopharmaceuticals must be analyzed and purified to reduce HCPs to an acceptable level. However, since the HCP mixture consists of a large number of protein species, which are unique to the specific host and not related to the intended recombinant protein, analysis of HCPs is not simple. They differ significantly in molecular mass, isoelectric point, hydrophobic properties, and structure. Moreover, they can undergo post-translational modifications that make quantification and characterization more difficult.
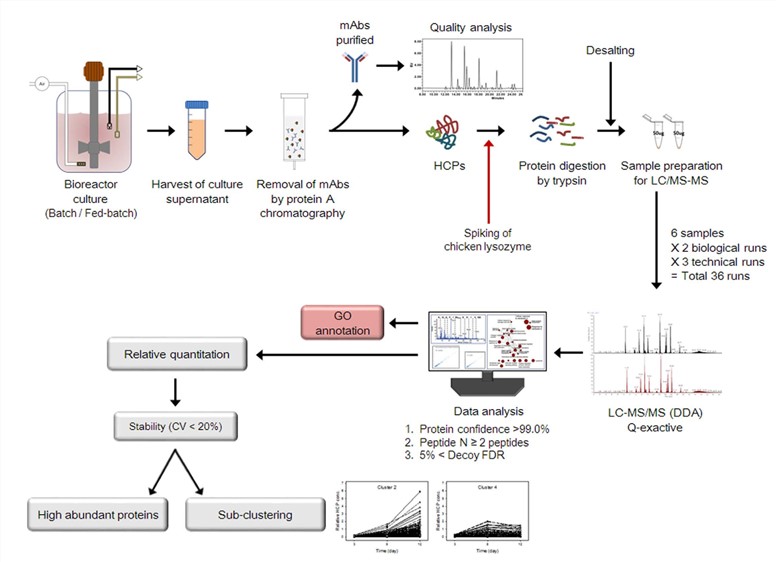 Fig. 1 Workflow used to characterize the quality attributes of mAbs and identify HCPs in the culture supernatants.1
Fig. 1 Workflow used to characterize the quality attributes of mAbs and identify HCPs in the culture supernatants.1
HCP Analytical Techniques
Creative Biolabs offers a wide spectrum of analytical techniques to characterize and quantify HCPs present in mAb products produced from different expression systems:
- Commercial ELISA kits: anti-HCP ELISA is a gold standard method used for HCP analysis. It is relatively easy to perform and is quantitative and sensitive. Besides, it can target a range of different proteins. It is applied during product development and process control.
- SDS-PAGE: This method provides good sensitivity, resolves multiple components, and provides qualitative information of HCPs.
- Western blot: This quantitative method can resolve multiple components and give relative molecular weight.
- Mass spectrometry (MS): MS-based methods are frequently employed for HCP detection due to the short processing time and high detection and quantification of proteins.
Features of Our Services
- Perform detailed characterization using a diverse range of analytical techniques
- Investigate the effect of impurities on antibody efficacy and performance using our established antibody function assays
- Eliminate the unwanted proteins if needed
- Tailored analytical services to meet different client requirements
Creative Biolabs offers a comprehensive range of analytical services around the identification and quantification of both process-related and product-related impurities to help understand your products and develop control strategies to reduce or remove them from your final product. If you are interested in our service, please contact us to discuss your requirements.
Reference
- Park, J. H.; et al. Proteomic analysis of host cell protein dynamics in the culture supernatants of antibody-producing CHO cells. Scientific reports. 2017, 7: 44246. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
