C1q Binding Assay
Creative Biolabs is dedicated to the ongoing exploration and advancement of therapeutic antibodies. Our dedicated scientific team developed an advanced C1q binding assay platform to deliver exceptional service in antibody function validation, focusing on a thorough analysis of Fc characteristics related to CDC activity.
C1q: Initiating Complement-Dependent Cytotoxicity in Antibody Therapy
Complement-dependent cytotoxicity (CDC) is a crucial non-cellular mechanism in antibody therapies against cancer and infections, particularly microbial invasions. This complex process involves over 20 complement proteins (C1-C9), initiated by C1 binding to the Fc regions of target-bound antibodies, triggering a cascade that culminates in target cell destruction via the membrane attack complex (MAC). Notably, the CDC can synergize with antibody-dependent cell-mediated cytotoxicity (ADCC) to amplify the overall killing efficacy of therapeutic antibodies. Consequently, assessing an antibody's Fc domain potency in recruiting complement and mediating CDC has become a vital step in early drug discovery for comprehensive Fc profiling and in vivo function prediction.
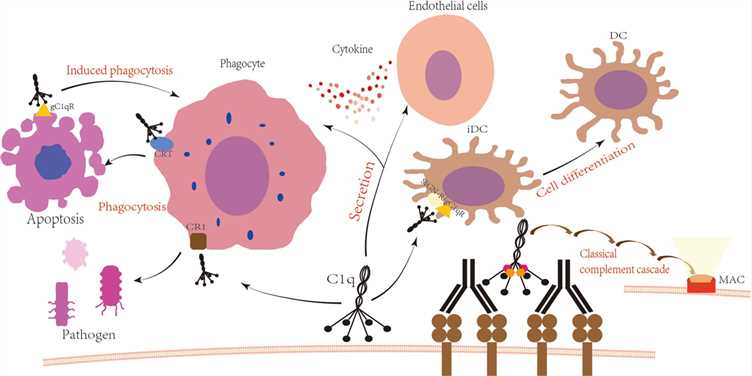 Fig.1 C1q participates in the immunological process.1
Fig.1 C1q participates in the immunological process.1
C1q Binding Assay at Creative Biolabs
Leveraging state-of-the-art facilities and extensive expertise in antibody functional mechanisms, Creative Biolabs presents its world-class C1q binding assay platform. Recognizing C1q as the primary initiator of the Complement-Dependent Cytotoxicity (CDC) pathway through its interaction with the antibody Fc region, we utilize the advanced surface plasmon resonance (SPR) technique to conduct comprehensive kinetic analysis. This powerful approach employs either recombinant C1q or purified human serum to provide a detailed understanding of the binding dynamics. Our highly versatile and flexible platform can be customized to accommodate diverse client requirements, offering a valuable complement to cell-based CDC assay by delivering crucial mechanistic insights into antibody-mediated effector functions.
Analyzable Antibody Subtypes
Our robust C1q binding assay platform is compatible with various sample types, which include:
- Serum
- Purified Antibodies
- Fc-Fusion Proteins
- Cell Culture Supernatants
- Plasma (less common)
Highlight Features
- Integrated Platform: Profiles Fc characteristics related to CDC activity.
- Kinetic Analysis: Employs surface plasmon resonance for comprehensive kinetic analysis.
- Flexible and Versatile: Can be tailored to meet different custom requirements.
- Mechanistic Insights: Serves as a valuable complement to cell-based CDC assays.
Data Display
Objective: The study aims to measure the binding affinity of sample-1 with C1q via the surface plasmon resonance method.
Assay Format: Binding and fitting curves between samples with C1q: Steady-state affinity model;
Results: The fitting curves for Sample-1 binding to C1q are shown in Fig.2. Using the steady-state affinity model, the captured Sample-1 can bind C1q with an affinity constant of 1.69x10-7 M.
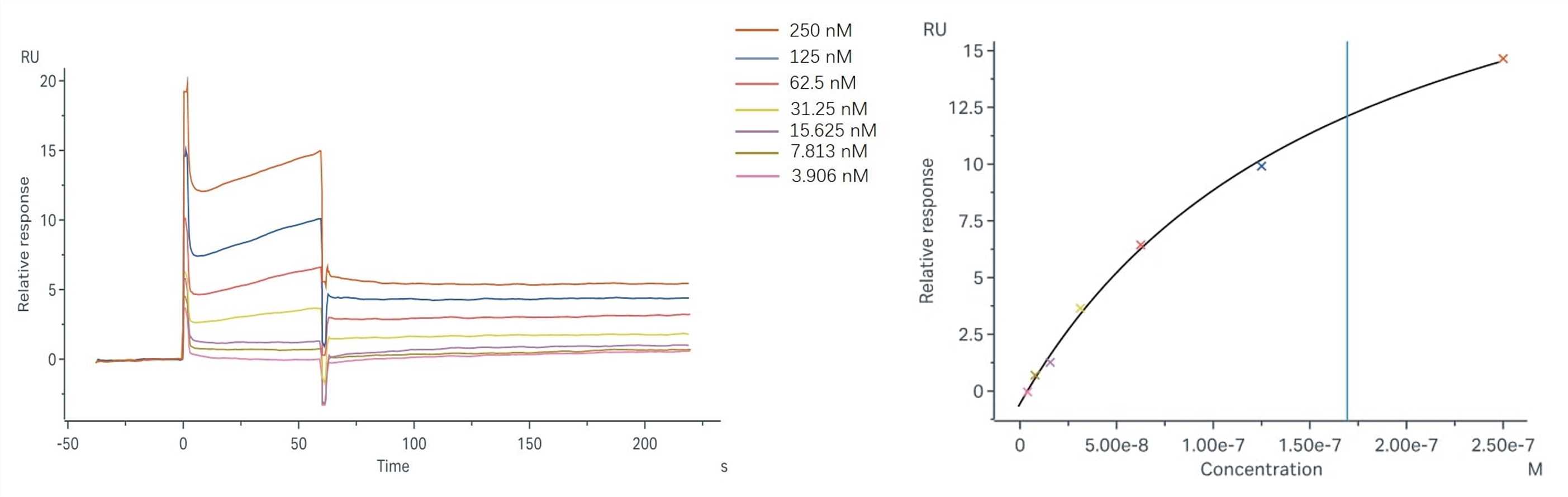 Fig.2 Binding and fitting curves between sample-1 with C1q at different concentrations.
Fig.2 Binding and fitting curves between sample-1 with C1q at different concentrations.
| Method | Ligand | Immobilized Level (RU) | Analyte | Analyte Conc. | KD (M) | Rmax (RU) | Chi² (RU²) | Fit method |
|---|---|---|---|---|---|---|---|---|
| CM4 | Sample-1 | 1521.1 | C1q | 3.906-250 nM | 1.69E-07 | 25.3 | 0.08 | Steady-state affinity |
Table 1 SPR result summary between sample-1 with C1q.
Q & A
-
Q1: What are the various approaches for C1q binding assays?
A1: C1q binding tests are routinely carried out utilizing ELISA, SPR, and flow cytometry. These strategies provide a variety of ways, each with its own set of benefits and concerns.
-
Q2: What are the advantages of using a C1q binding assay compared to cell-based CDC assays?
A2: C1q binding assays can offer a more direct and mechanistic understanding of the initial interaction. They can also be faster, more easily standardized, and less influenced by cell-specific factors. However, they don't fully recapitulate the entire CDC process.
-
Q3: What is the required amount of sample for the assay?
A3: Generally, the required amount of sample for a C1q binding assay can vary depending on the specific assay platform and the nature of the sample. Please contact our team for detailed information. Here are some common ranges for reference:
- Serum samples: The typical minimum volume for serum samples often ranges from 0.1 mL to 0.6 mL, with preferred volumes sometimes being around 1 mL to 2 mL. Some labs might require even more (e.g., 3 mL or 7 mL).
- Purified Antibodies/Fc-Fusion Proteins: If you are submitting purified protein for direct C1q binding analysis, the required amount is usually specified in terms of concentration and volume. This could range from micrograms to milligrams, depending on the assay and the number of concentrations being tested.
Related Services
Leveraging a comprehensive Fc assessment portfolio, Creative Biolabs offers an integrated service for characterizing antibody Fc regions and specializes in Fc engineering to generate antibodies with improved cytotoxic function. Notably, the synergistic effect of combining advantageous FcγR and C1q mutations within Fc domains has been demonstrated, leading to optimized antibodies with superior ADCC and CDC bioactivities, highlighting a significant opportunity for advancing next-generation antibody therapeutics.
If you are interested in our C1q binding assay, please click the link to get in touch with us or email us an inquiry.
Reference
- Zhang, Wenjie, Yuan Chen, and Hui Pei. "C1q and central nervous system disorders." Frontiers in Immunology 14 (2023): 1145649. Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only.
