Carbon Tetrachloride (CCL₄) induced Acute Liver Injury Modeling & Pharmacodynamics Service
Creative Biolabs offers a variety of well-established models for evaluating drug efficacy in acute liver injury. These models enable researchers to test new therapies, understand mechanisms of liver damage, and provide insights into liver regeneration and repair.
Introduction
Acute liver injury (ALI) is a rapid-onset liver condition characterized by hepatocyte damage, inflammation, and sometimes liver failure. It can be caused by various factors, including drugs, toxins, viruses, and alcohol consumption. If left untreated, ALI can progress to acute liver failure, which requires immediate medical intervention. The severity of ALI varies depending on the cause and extent of liver damage, and it can lead to significant morbidity and mortality if not managed appropriately. The use of preclinical models for acute liver injury plays a crucial role in understanding the pathophysiology of ALI and evaluating therapeutic interventions.
Disease Models and Applications
The CCl4 induced Acute Liver Injury Model is created by administering carbon tetrachloride (CCl4) to rodents and NHPs, typically via intraperitoneal or oral routes. Once metabolized by the liver, CCl4 generates free radicals, leading to hepatocyte membrane damage, oxidative stress, and inflammation. This model is highly regarded for simulating acute liver damage, as it induces marked liver injury within hours to days. The primary advantage of this model is its ability to mimic both hepatocyte necrosis and inflammation, making it suitable for evaluating hepatoprotective drugs and liver regeneration therapies. However, while the CCl4 model provides insights into acute injury, it does not fully replicate chronic liver disease or the progressive stages leading to cirrhosis, which limits its application for long-term disease studies. Despite this, it remains one of the most widely used models for understanding liver toxicity and inflammation.
- Simulates: The CCl4 induced Acute Liver Injury Model simulates acute hepatotoxicity and liver inflammation, providing an essential tool for studying drug induced liver injury (DILI), acute liver failure, and oxidative stress-mediated liver damage. It also mimics the inflammatory response involved in liver injury.
- Evaluates Drugs: The model is extensively used to evaluate hepatoprotective agents, anti-inflammatory drugs, and compounds aimed at reducing liver damage. It is useful for assessing the potential of drugs that mitigate acute liver toxicity, reduce inflammation, and promote liver regeneration.
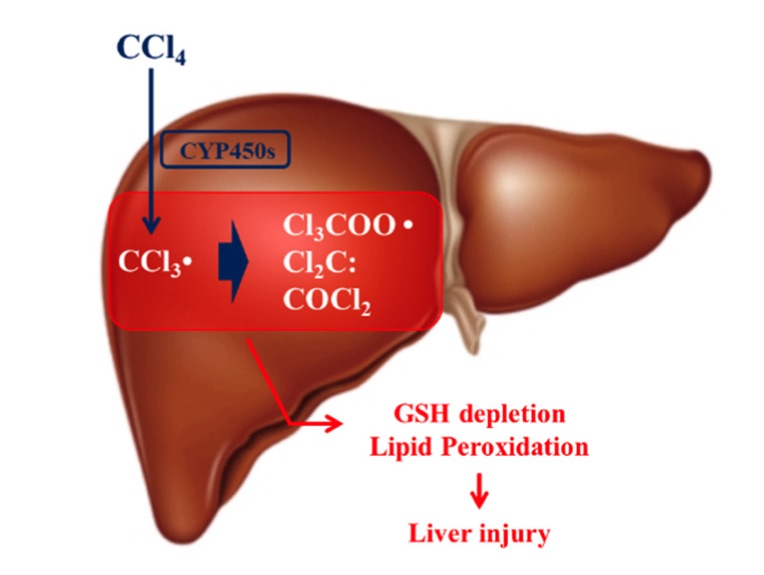 Fig. 1 Mechanism of carbon tetrachloride (CCl4) hepatotoxicity.1
Fig. 1 Mechanism of carbon tetrachloride (CCl4) hepatotoxicity.1
Measurements
We offer a variety of measurements for evaluating drug efficacy in the CCl4 induced Acute Liver Injury Model, using advanced technologies such as:
- General observations: Body weight, mortality rate, liver enlargement, stool consistency, and gastrointestinal bleeding.
- Histological analysis: Hematoxylin and eosin (H&E) staining for liver architecture, hepatocyte necrosis, and inflammation.
- Immunohistochemistry: Assessment of immune cell infiltration (e.g., T-cells, macrophages) in liver tissues.
- Biomarkers: Measurement of serum liver enzymes (ALT, AST), bilirubin levels, and other liver injury markers.
- Cytokine profiling (e.g., ELISA): Levels of inflammatory cytokines such as TNF-α, IL-6, and IL-1β.
- Gene/protein expression profiling: RT-qPCR and Western blot techniques for assessing markers of oxidative stress, liver injury, and fibrosis.
Additionally, we provide expert assistance in experimental design, data analysis, and model selection to ensure the most appropriate approach for your research objectives.
Related Services
Alongside the CCl4 induced Acute Liver Injury Model, we also offer alternative acute liver injury models induced by various agents such as acetaminophen (APAP), ischemia-reperfusion injury, and ethanol. These models allow for a comprehensive understanding of liver injury and repair mechanisms.
- Concanavalin A (Con A) induced Acute Liver Injury Model
- Polyinosinic: Polycytidylic Acid induced Acute Liver Injury Model
- Acetaminophen (APAP) induced Acute Liver Injury Model
- Alcohol induced Acute Liver Injury Model
- Ischemia-Reperfusion induced Liver Injury Model
- Alpha-Naphthylisothiocyanate (ANIT) induced Acute Liver Injury Model
- DDC (3,5-Diethoxycarbonyl-1,4-Dihydrocollidine) induced Acute Liver Injury Model
Advantages
- Comprehensive Model Portfolio: We offer a variety of established and customized models for studying acute liver injury and evaluating drug efficacy.
- Expertise in Drug Evaluation: Our scientific team has extensive experience in designing experiments to assess hepatoprotective and anti-inflammatory drug candidates.
- Advanced Technologies: We use state-of-the-art technologies for biomarker analysis, gene/protein expression profiling, and histological assessments.
- Tailored Approach: We provide tailored support, from model selection to data analysis, ensuring a personalized approach to your research needs.
- Reliable Results: Our models are scientifically validated, offering reproducible results that support reliable drug development processes.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. How long does the CCl4 induced Acute Liver Injury Model take to show liver damage?
Liver injury can be observed within 24-48 hours after CCl4 administration, with peak damage typically seen around 72 hours.
-
2. Can this model be used to evaluate treatments for chronic liver diseases?
While primarily designed for acute liver injury, modifications to the model can be made to assess the early stages of fibrosis and chronic injury.
-
3. What species are used in the CCl4 induced Acute Liver Injury Model?
The model is commonly used in rodents (rats and mice), but other species can be considered depending on your specific needs.
-
4. Do you assist with data analysis and interpretation?
Yes, our team provides full support in data analysis, ensuring that your results are interpreted accurately and effectively for your research.
Published Data
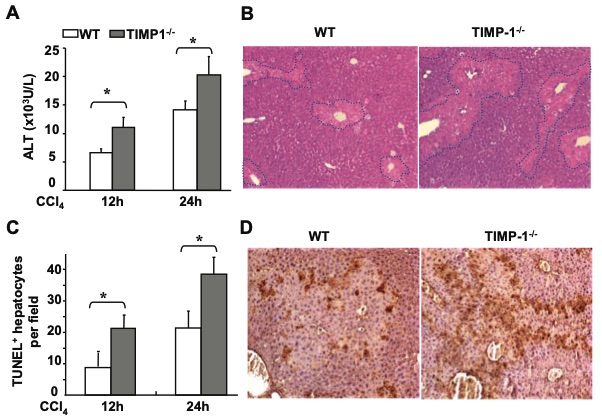 Fig. 2 TIMP-1-/- mice are more susceptible to CCl4 induced acute liver injury.2
Fig. 2 TIMP-1-/- mice are more susceptible to CCl4 induced acute liver injury.2
Previous studies have suggested that TIMP-1 plays a significant role in liver fibrosis and regeneration; however, its function during hepatocellular injury remains unclear. To investigate this, an experiment was conducted to compare the acute liver injury induced by a single dose of CCl4 injection in TIMP-1-/- mice and wild-type mice. All animals survived after the CCl4 challenge. As shown in Figure 2A, TIMP-1-/- mice exhibited significantly higher levels of serum ALT and AST compared to wild-type mice following the acute CCl4 injection. In line with the elevated serum ALT levels, histological analysis revealed that TIMP-1-/- mice had larger areas of necrosis, as assessed by H&E staining (Figure 2B). The acute CCl4 administration caused necroinflammatory liver damage primarily localized in the pericentral regions in wild-type mice. In contrast, TIMP-1-/- mice displayed a more severe liver injury with widespread necrotic hepatocyte foci. Additionally, TUNEL assay results indicated a significantly higher number of apoptotic hepatocytes in TIMP-1-/- mice compared to wild-type mice (Figures 2C-D).
References
- Jeong, Tae Bin et al. "Weaning Mice and Adult Mice Exhibit Differential Carbon Tetrachloride induced Acute Hepatotoxicity." Antioxidants (Basel, Switzerland) vol. 9,3 201. 1 Mar. 2020, DOI:10.3390/antiox9030201. Distributed under an Open Access license CC BY 4.0, without modification.
- Wang, Hua et al. "Tissue inhibitor of metalloproteinase 1 (TIMP-1) deficiency exacerbates carbon tetrachloride induced liver injury and fibrosis in mice: involvement of hepatocyte STAT3 in TIMP-1 production." Cell & bioscience vol. 1,1 14. 4 Apr. 2011, DOI:10.1186/2045-3701-1-14. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
