CDC Assay Service
Long drug development cycles and challenges in antibody therapeutic characterization can significantly hinder progress. Creative Biolabs offers an advanced Complement-Dependent Cytotoxicity (CDC) Assay service, leveraging innovative cell-based assay technology and robust screening platforms to help you accelerate drug discovery and develop highly specific and potent antibody-based therapeutics. Our solutions streamline preclinical assessment, providing critical insights into your molecule's mechanism of action.
The Crucial Role of CDC in Therapeutic Antibody Development
The precise characterization of therapeutic antibodies is paramount in modern biopharmaceutical development. Complement-dependent cytotoxicity (CDC) is a critical immune effector mechanism by which antibodies can specifically eliminate target cells, playing a vital role in therapies for cancer and autoimmune diseases. Understanding and harnessing CDC activity is essential for developing safe and effective antibody therapeutics. While novel gene therapies show immense promise, the broader field of biopharmaceuticals constantly demands robust methods to assess immune responses, making reliable CDC assays indispensable for predicting clinical efficacy and ensuring drug safety.
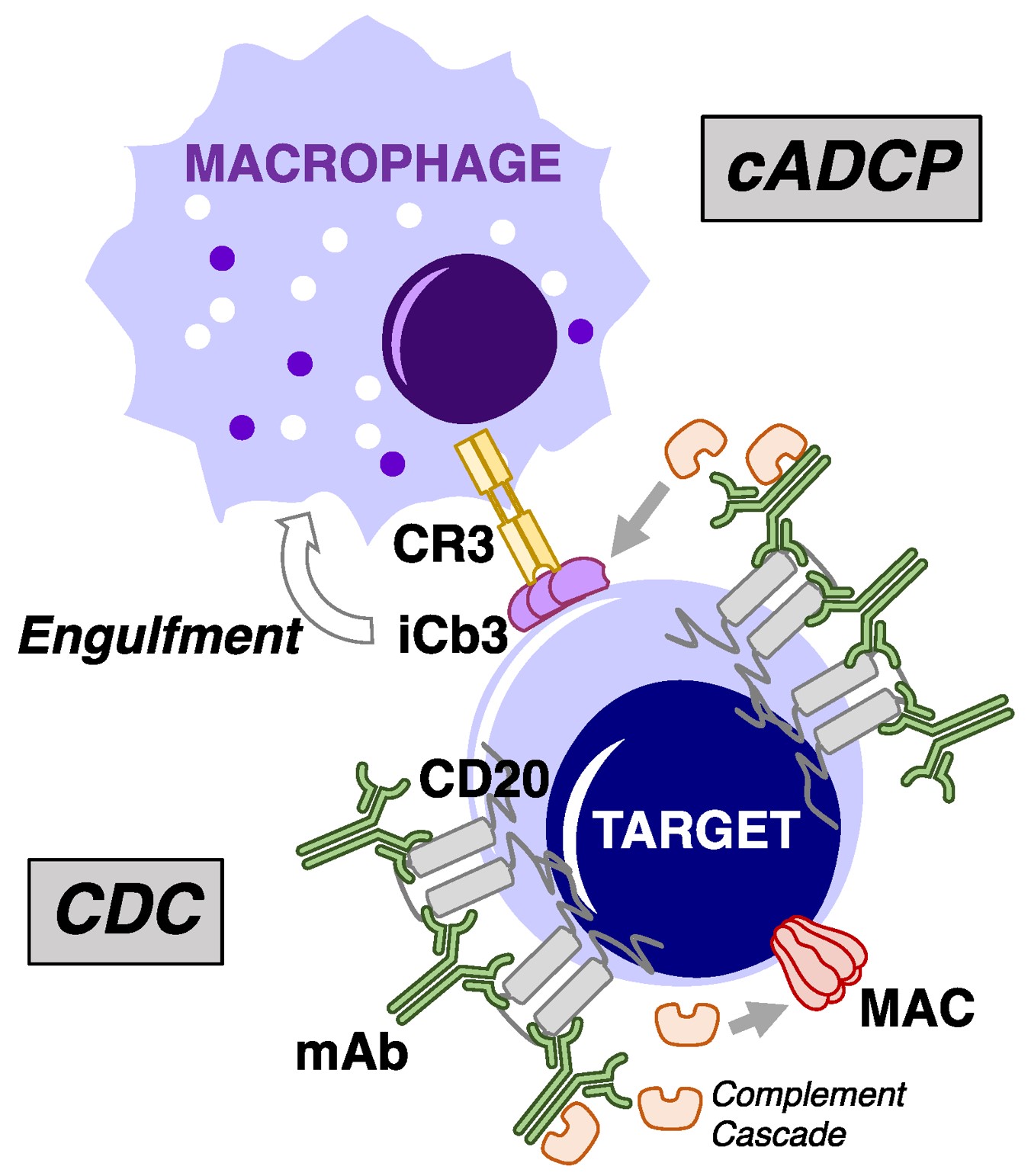 Fig.1 Overview of cytotoxic processes underpinning mAb-mediated complement fixation.1
Fig.1 Overview of cytotoxic processes underpinning mAb-mediated complement fixation.1
CDC Assay at Creative Biolabs
Creative Biolabs' CDC Assay service delivers comprehensive solutions for characterizing your antibody candidates, providing crucial insights into their complement-activating potential. Our services are designed to meet the rigorous demands of preclinical development, offering specific deliverables, a clear workflow, and an unwavering commitment to quality. We employ an advanced, non-radioactive bioluminescent assay that quantifies cell lysis by measuring released GAPDH, providing highly sensitive and accurate results typically within 4 to 8 weeks, depending on project complexity. This methodology, combined with ICH-compliant standards, rigorous quality control, and the expertise of our scientists, guarantees reliable and reproducible data essential for advancing your therapeutic candidates.
Our Workflow
01Step1. Project Consultation & Experimental Design
02Step2. Target Cell Line Preparation
03Step3. Antibody & Complement Incubation
04Step4. Cytotoxicity Detection & Measurement
05Step5. Data Analysis & Reporting
Service Highlights
- High Sensitivity & Accuracy: Detect even subtle CDC activity with robust, reproducible results.
- Non-Radioactive Methodology: Eliminate safety concerns and disposal issues associated with traditional isotopic assays.
- High-Throughput Capability: Efficiently screen numerous antibody candidates, accelerating lead selection.
- Customizable Assays: Flexible experimental design tailored to your specific antibody and target.
- High Quality: Assays prequalified for precision, accuracy, and linearity, meeting regulatory standards for potency and stability studies.
Key Elements to Achieve Highly Efficient CDC Assay
Achieving a highly efficient and accurate CDC assay hinges on carefully considered experimental design and high-quality reagents. Creative Biolabs prioritizes these key elements to deliver optimal results for your projects.
|
We can utilize frozen cells provided by customers upon request. If you haven't identified your ideal target cell line, our extensive expertise allows us to recommend the most appropriate one from our library of over a hundred well-qualified tumor cell lines. Our scientists also possess vast experience in designing various disease models to ensure the relevance of your assay. |
|
We offer high-quality complement systems, primarily human serum, which adhere to strict QC standards under regulatory guidelines. Alternatively, we can provide serum from various rodent and non-rodent species to suit the specific requirements of your research. |
|
Our laboratory supports multiple endpoints and readout formats. Typical cell lysis detection methods include measuring LDH/ATP/GAPDH release, radioactivity measurement (though our primary method is non-radioactive), and FACS screening. We are also adept at designing alternative assay readouts tailored to your custom requests. |
Case Study
- Objective: The study aims to characterize samples via the CDC dose-response assay.
- Assay Format:
|
|
|
|
|
|
- Results: In this study, the results of the CDC dose-response study showed that the EC50 value of sample-X was 0.1011 μg/mL.
| log(agonist) vs. response -- Variable slope (four parameters) | |||||
| Best-fit values | |||||
| Bottom | Top | LogEC50 | HillSlope | EC50 | Span |
| -1.751 | 73.07 | -0.9951 | 1.28 | 0.1011 | 74.82 |
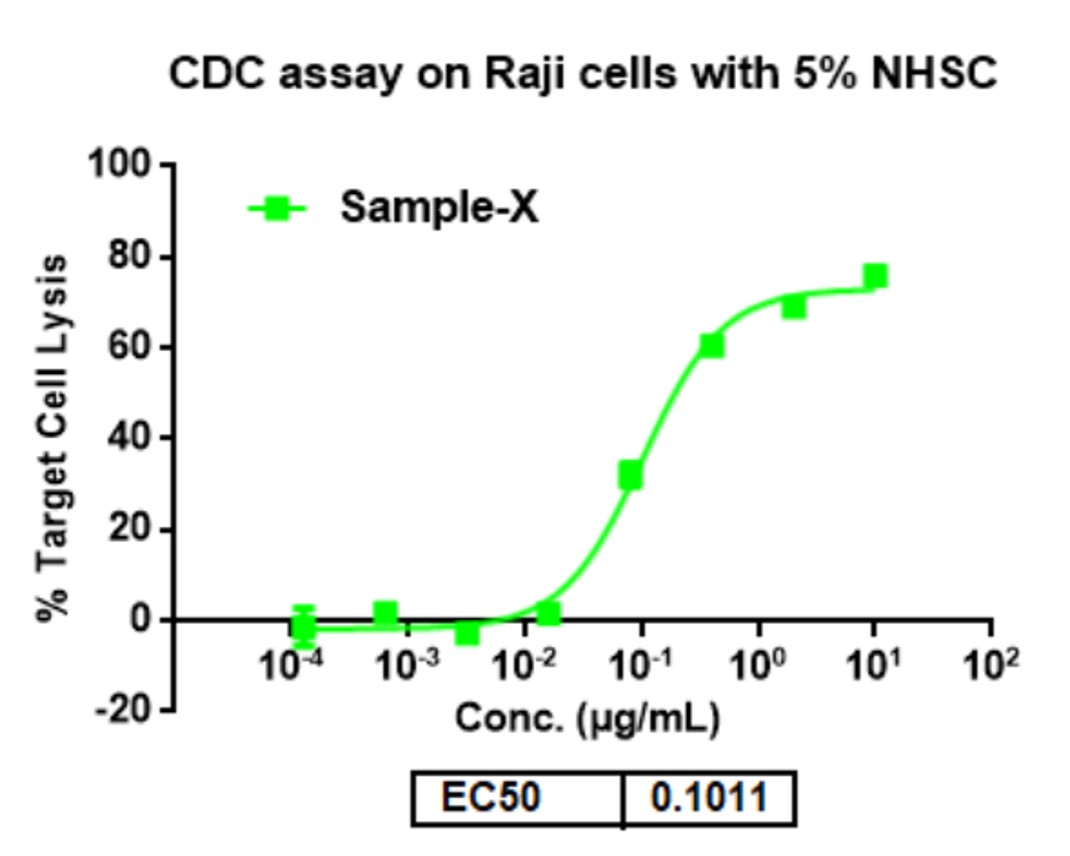 Fig.2 Best-fit values summary for the CDC dose-response study of sample-X.
Fig.2 Best-fit values summary for the CDC dose-response study of sample-X.
FAQs
-
Q1: What types of antibodies can Creative Biolabs' CDC assay evaluate?
A1: Our CDC assay platform is highly versatile and can evaluate a wide range of antibody formats, including conventional monoclonal antibodies (mAbs), bispecific antibodies, and antibody-drug conjugates (ADCs). We customize the assay parameters to suit the unique characteristics of your therapeutic molecule.
-
Q2: Can I provide my own target cells and complement source for the assay?
A2: Absolutely. We welcome clients to provide their specific target cell lines and preferred complement sources (e.g., human serum, animal serum). Our team will work with you to ensure these materials are optimally integrated into the assay design for the most relevant results.
-
Q3: What precautions are taken to ensure the quality and consistency of the CDC assay results?
A3: Creative Biolabs adheres to strict quality control standards throughout the entire CDC assay process. This includes using quality-controlled complement sources, meticulously validating cell line performance, and employing standardized protocols. Our assays are prequalified according to ICH guidelines for precision, accuracy, and linearity, ensuring robust and reliable data for your regulatory submissions.
-
Q4: Is it possible to combine the CDC assay with other effector function analyses?
A4: Yes, many clients choose to combine CDC assays with other effector function analyses, such as Antibody-Dependent Cell-mediated Cytotoxicity (ADCC) and Antibody-Dependent Cellular Phagocytosis (ADCP) assays. This provides a comprehensive understanding of your antibody's immunomodulatory potential. Please contact us to discuss an integrated approach for your project.
Related Sections
Creative Biolabs offers unrivaled experience and cutting-edge technology for CDC Assay services, allowing you to properly define your antibody therapies.
- Antibody-Dependent Cell-mediated Cytotoxicity (ADCC) Assay
- Antibody-Dependent Cellular Phagocytosis (ADCP) Assay
- FcγRIIa Binding Assay
- FcγRI Binding Assay
- FcγRIIIa Binding Assay
Are you ready to accelerate your antibody therapy research with Creative Biolabs' sophisticated CDC Assay services? Please contact us, and our team of experienced scientists is eager to discuss your specific project needs and provide tailored solutions.
Reference
- Zent, Clive S et al. "Complement Activation in the Treatment of B-Cell Malignancies." Antibodies (Basel, Switzerland) vol. 9,4 68. 1 Dec. 2020, doi:10.3390/antib9040068. Distributed under an Open Access License CC BY 4.0, without modification.
For Research Use Only.
