FcRn Binding Assay
Are you currently encountering difficulties in optimizing the pharmacokinetic properties of your therapeutic antibodies or Fc-fusion proteins? Creative Biolabs' FcRn binding assay service facilitates the acceleration of drug development through the provision of precise and reliable assessments of FcRn interactions, which are crucial for enhancing drug efficacy and extending half-life.
Background
The neonatal Fc receptor (FcRn) regulates the serum half-life of IgG antibodies and albumin. This connection is critical to the effectiveness of antibody-based therapies. Accurate characterization of FcRn binding is essential for optimizing the pharmacokinetic properties, improving drug efficacy, and reducing dosage frequency. Research and development in this area directly support the development of more effective and patient-friendly biopharmaceuticals.
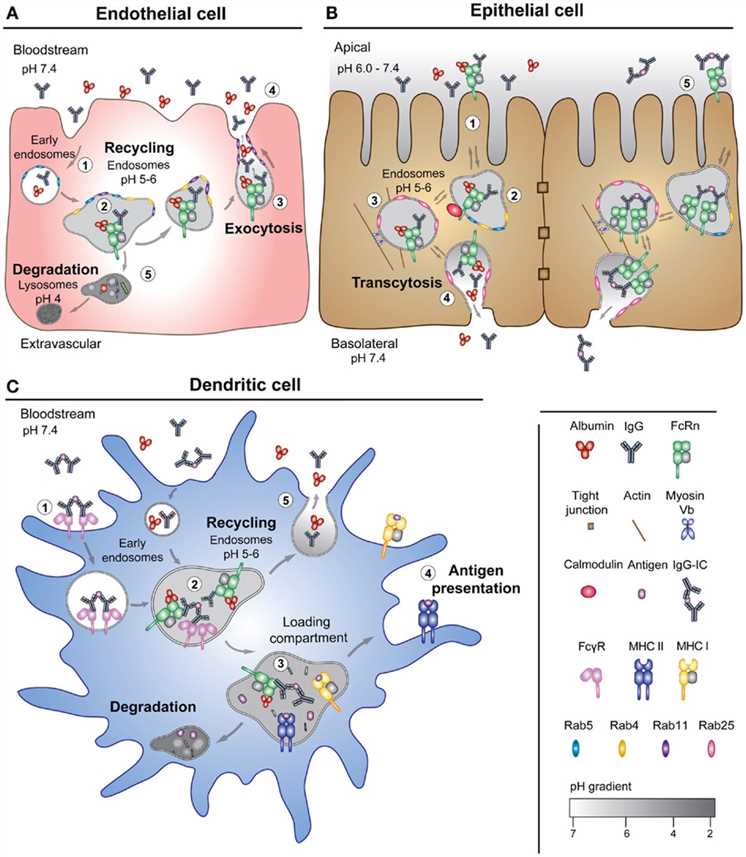 Fig.1 FcRn-mediated pathways for transportation.1
Fig.1 FcRn-mediated pathways for transportation.1
FcRn Binding Assay at Creative Biolabs
Creative Biolabs offers a comprehensive FcRn Binding Assay service designed to precisely evaluate the interaction between your therapeutic molecules and FcRn. This service provides critical data for optimizing drug candidates and predicting in vivo behavior. Specifically, it enables researchers to gain a deeper understanding of how their molecules interact with FcRn under various physiological conditions. This understanding is paramount in the early stages of drug development, allowing for the selection of lead candidates with the most favorable pharmacokinetic profiles. Furthermore, the data generated from our assays can be used to refine molecular structures, optimize dosing regimens, and ultimately, improve the therapeutic efficacy and safety of the final drug product. By accurately predicting in vivo behavior, our service contributes to a more efficient and cost-effective drug development process, reducing the risk of late-stage failures due to suboptimal pharmacokinetics.
Our Service Workflow
Assay Design and Optimization: We tailor the assay format (e.g., ELISA, SPR, BLI) based on client needs and sample characteristics, including selecting appropriate FcRn variants (human, mouse, etc.) and optimizing buffer conditions.
FcRn Protein Preparation: We utilize advanced recombinant protein expression and purification techniques to produce high-quality, biologically active FcRn protein via cell line selection, culture optimization, and multi-step chromatography.
Sample Preparation and Labeling (if required): Client-provided samples are prepared for the selected assay, sometimes involving labeling with a fluorescent dye or biotin. We perform quality control checks to ensure sample integrity and labeling efficiency.
Binding Assay Execution: The assay is performed under controlled conditions, including temperature, pH, and incubation time. Multiple replicates ensure data reproducibility. Automation enhances throughput and reduces variability.
Data Acquisition and Analysis: Binding data (e.g., absorbance, fluorescence, or SPR signals) is acquired using specialized instrumentation. We use validated software for data analysis, including curve fitting and determining binding parameters.
Report Generation and Delivery: A comprehensive report is generated, detailing the assay methodology, raw and processed data, data interpretation, quality control data, and relevant observations.
Available Detection Methods at Creative Biolabs
Creative Biolabs employs a variety of advanced detection methods to comprehensively analyze FcRn binding interactions. These approaches include, but are not limited to the following:
- ELISA
- Flow cytometry
- Surface plasmon resonance / Bio-layer interferometry: real-time, label-free full kinetic analysis.
Analyzable Antibody Subtypes
Creative Biolabs' FcRn binding assay service is designed to accommodate a wide range of antibody subtypes and Fc-fusion proteins, including but not limited to:
- IgG1, IgG2, IgG3, and IgG4
- Fc-fusion proteins
- Modified antibody formats (e.g., Fab fragments, scFvs, bispecific antibodies)
Attractive Advantages
- High Sensitivity and Specificity
- Quantitative Binding Data
- Customizable Assay Formats
- Fast Turnaround Time
- Expert Consultation and Support
Case Study
Objective: Use the surface plasmon resonance technique to determine Sample-1's binding affinity with FcRn.
Assay Format: Binding and fitting curves for the Sample-1 and FcRn using the steady-state affinity model.
Results: The fitting curves for Sample-1 binding to human FcRn are shown in the following. Using the steady-state affinity model, the captured FcRn can bind Sample-1 with an affinity constant of 1.20 x 10-7 M.
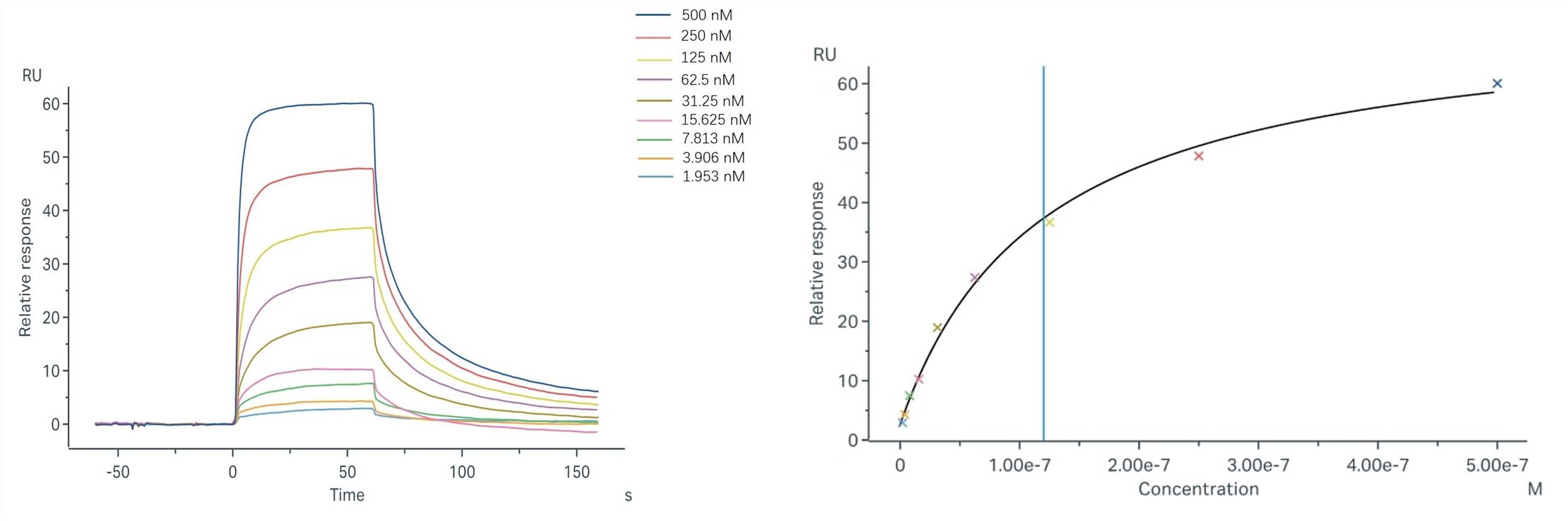 Fig.2 Binding and fitting curves between Sample-1 with FcRn at different concentrations.
Fig.2 Binding and fitting curves between Sample-1 with FcRn at different concentrations.
| Method | Ligand | Capture Level (RU) | Analyte | Analyte Conc. | ka (1/Ms) | kd (1/s) | KD (M) | Rmax (RU) | Chi² (RU²) | Fit method |
|---|---|---|---|---|---|---|---|---|---|---|
| His Capture | Human FcRn | 54.4 | Sample-1 | 1.953-500 nM | NA | NA | 1.20E-07 | 69.4 | 2.19 | Steady-state affinity |
Table 1 SPR result summary between Sample-1 with FcRn.
Related Sections
Furthermore, Creative Biolabs provides a specialized Fc engineering service focused on enhancing in vivo half-life. By employing targeted amino acid point mutagenesis within the FcRn binding site, we aim to achieve increased binding at pH 6.0 and reduced dissociation at pH 7.2. This strategy facilitates optimized clinical dosing, and our adaptable, sensitive FcRn binding evaluation platform accelerates the selection of promising candidates during early discovery, ultimately improving the likelihood of success in later development stages.
For additional information, please contact us or submit an inquiry directly.
Reference
- Sand, Kine Marita Knudsen, et al. "Unraveling the interaction between FcRn and albumin: opportunities for design of albumin-based therapeutics." Frontiers in Immunology 5 (2015): 118912. Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only.
