FcγR Binding Assays
Creative Biolabs possesses significant expertise in analyzing and characterizing antibody Fc functions, particularly their interaction with Fc-gamma receptors (FcγRs) on immune cells, which can trigger immune responses like complement fixation. Our adaptable technology platform delivers comprehensive Fc receptor profiling services for custom antibodies, with a strong focus on in-depth FcγR binding analysis to enhance your research.
FcγR Introduction
Therapeutic antibodies often exert their effects by engaging immune effector mechanisms through their Fc regions, which bind to specific Fcγ receptors or complement proteins. Expressed on leukocytes, FcγRs are categorized into three classes (I, II, and III) with varying affinities and functions; for example, FcγRIIb inhibits while others activate via ITAM signaling. Receptor polymorphisms and antibody glycosylation impact binding. This Fc-FcγR interaction triggers crucial immune responses like effector cell recruitment, cytokine release, B cell activation, and cytotoxicity, with FcγRIIIa (CD16a) driving ADCC and FcγRIIa (CD32a) mediating ADCP.
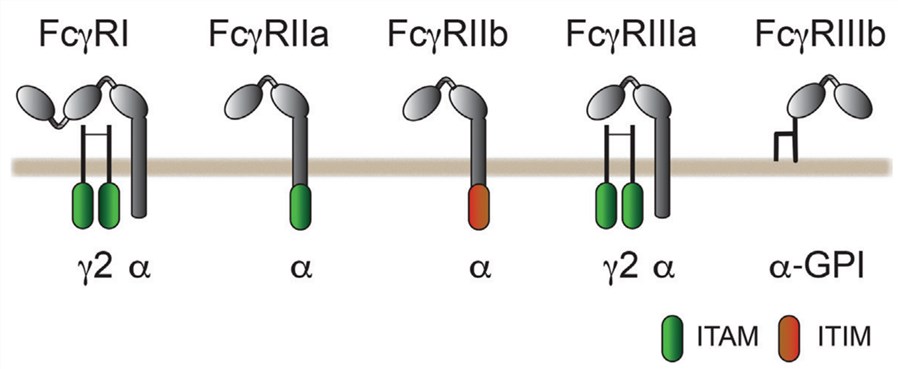 Fig.1 The classification of human FcγRs.1
Fig.1 The classification of human FcγRs.1
FcγR Binding Assays at Creative Biolabs
Creative Biolabs offers comprehensive FcγR binding assays to characterize the interaction between antibody Fc regions and various Fc gamma receptors (FcγRs). Utilizing techniques like ELISA, flow cytometry, and surface plasmon resonance (SPR), we provide detailed kinetic data, including association and dissociation rates and affinity constants, for different IgG subtypes and Fc fusion proteins. These assays help researchers understand the mechanism of action of therapeutic antibodies and optimize their development by analyzing their potential to elicit immune responses through FcγR engagement.
Diverse FcγR Targets
Harnessing cutting-edge technologies and the expertise of our seasoned scientists, Creative Biolabs presents a versatile and dependable platform for in-depth analysis of FcγR binding profiles. Our comprehensive service provides researchers with the tools to thoroughly investigate antibody Fc region interactions with a wide array of FcγR targets (list below), complete with relevant positive controls.
Selective Quantification Approaches
Creative Biolabs is capable of performing FcγR binding assays for various IgG antibody isotypes (IgG1, IgG2, IgG3, and IgG4) or Fc fusion proteins with different approaches, including but not limited to:
Multi-species Compatibility Assays
Creative Biolabs utilizes advanced surface plasmon resonance (SPR) technology for precise FcγR binding assessments. Our services encompass a range of assays to evaluate the FcγR binding capabilities of humanized antibodies. Furthermore, we offer FcγR binding assays for various species, enabling the screening of non-humanized antibodies and the investigation of cross-species reactivity.
| Human |
Human FcγRI / CD64 Human FcγRIIB/C / CD32b/c Human FcγRIIA / CD32a (H167) Human FcγRIIA / CD32a (R167) Human FcγRIIIA / CD16a (V176) Human FcγRIIIA / CD16a (F176) Human FcγRIIIB / CD16b (NA1) Human FcγRIIIB / CD16b (NA2) Human Fc epsilon RII/ CD23 Human Fc epsilon RI alpha Human FcRn / FCGRT&B2M |
Rat |
Rat FcγRI / CD64 Rat FcγRIIA / CD32a Rat FcγRIIB / CD32b Rat FcRn / FCGRT&B2M Heterodimer Protein |
Cynomolgus |
Cynomolgus FcγRI / CD64 Cynomolgus FcγRIIA / CD32a Cynomolgus FcγRIIB / CD32b Cynomolgus FcγRIII / CD16 Cynomolgus / Rhesus macaque FcRn / FCGRT&B2M Heterodimer Protein |
| Mouse |
Mouse FcγRI / CD64 Mouse FcγRIIB / CD32b Mouse FcγRIII / CD16 Mouse FcγRIV / CD16-2 Mouse FcRn / FCGRT&B2M Heterodimer Protein |
Rabbit | Rabbit FcRn / FCGRT&B2M Heterodimer Protein | Canine |
Canine FcγRI / CD64 Canine FcRn / FCGRT&B2M Heterodimer Protein |
Service Advantages
- A variety of Selective FcγR targets
- Multi-species compatibility
- Advanced technology support
- One-stop customized service
Related Sections
Furthermore, we offer specialized services for evaluating FcRn and C1q binding interactions. Moreover, combining FcγR binding data with conventional cytotoxicity assays provides a thorough insight into the mechanism of action of an antibody. Understanding the possibility of differences in cytotoxicity results from in vitro to in vivo, particularly due to the influence of endogenous human serum IgG, it is essential to enhance Fc avidity and affinity for FcγRs to boost clinical effectiveness. To tackle this challenge, Creative Biolabs provides specialized Fc engineering services, employing genetic manipulation and glycoform modification to accurately adjust binding affinities for targeted FcγRs.
Customer Speaking
- Au***a 21/Jul/2024 Efficient and High-Quality Result Delivery! ⭐⭐⭐⭐⭐
We were pleased with Creative Biolabs' FcγR binding test service. The thorough study provided kinetic data that helped us comprehend our lead antibody candidate's function. The professional and well-coordinated crew provided valuable project insights. The flexible framework allowed us to study several FcγR variations, and obvious positive controls reinforced our confidence in the findings. We were impressed by the speedy turnaround and the clear, concise, and ready-to-use final report. Creative Biolabs is a crucial antibody development partner.
- Li***y 16/Sep/2023 Flexible and Highly Reproducible! ⭐⭐⭐⭐⭐
Creative Biolabs offered an original and dependable platform, and their knowledge of FcγR binding tests helped me choose them. Important for the later functional testing, the results revealed consistency and reproducibility across investigations. One big benefit was changing the test parameters to match our antibody and study question. For research organizations seeking thorough FcγR binding studies, I strongly suggest Creative Biolabs.
Creative Biolabs: Your Reliable Partner
At Creative Biolabs, our experienced scientists excel in customizing diverse antibody functional assays, and we are dedicated to supporting our global clients in their innovative antibody research. Our advanced assay platform guarantees accurate and sensitive results, seamlessly integrating with automated high-throughput systems. For further details, please reach out to us or submit an inquiry.
Reference
- Rosales, Carlos, and Eileen Uribe-Querol. "Neutrophil activation by antibody receptors." Neutrophils. IntechOpen, 2018. Distributed under Open Access License CC BY 3.0, without modification.
For Research Use Only.
