FcγRIIa Binding Assay
Creative Biolabs offers an extensive suite of sophisticated assays designed to provide our global clientele with a thorough understanding of the Fc binding characteristics of their promising antibody candidates with a specific emphasis on detailed analysis of interactions with FcγRIIa. Our comprehensive portfolio enables in-depth characterization of binding affinity, kinetics, and specificity, which is crucial for predicting in vivo efficacy and guiding the optimization of antibody therapeutics.
FcγRIIa Overview
FcγRIIa (CD32a), a low-affinity single-chain FcγR that is stimulatory but expressed on a variety of leukocytes, directly signals through ITAMs to facilitate cell-specific functions such as ADCP in macrophages, respiratory burst in neutrophils, platelet activation, and antigen presentation in dendritic cells. It frequently functions in conjunction with other stimulatory FcγRs, such as FcγRIIIa. Most importantly, a histidine/arginine polymorphism at position 131 produces three allelic variations (HH/RR/HR) with different antibody Fc binding affinities, therefore stressing the need to address FcγRIIa genotypes when designing antibody treatments with immune effector mechanisms.
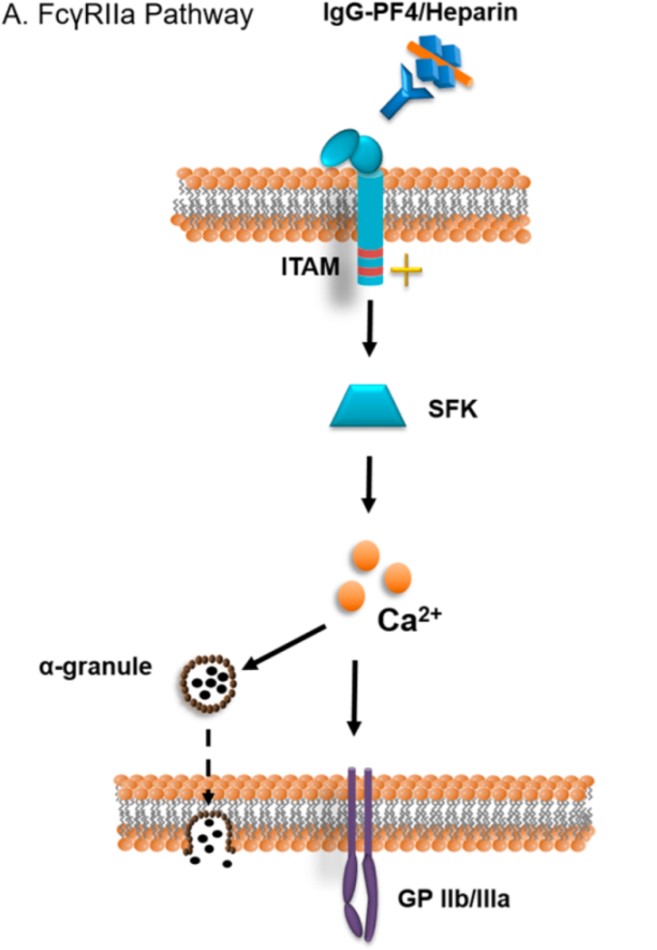 Fig.1 The FcγRIIa receptor's signaling pathway.1
Fig.1 The FcγRIIa receptor's signaling pathway.1
Our FcγRIIa Binding Assay
To ensure a detailed comprehension of the interaction between your custom antibodies and FcγRIIa, we have developed an advanced and all-encompassing testing platform, utilizing our in-depth knowledge of Fc receptor biology. We adopt a collaborative methodology, initiating an in-depth consultation to identify your unique requirements and customize the assay design for maximum effectiveness. After thorough implementation using our cutting-edge technologies, you will obtain a detailed report that includes binding kinetics, affinity measurements, and, if relevant, polymorphism-specific analysis, all presented in a clear and concise manner. Throughout the process, our knowledgeable professionals offer committed assistance and specialized insights, guaranteeing dependable collaboration and the provision of accurate, high-caliber data to enhance your antibody development projects.
Selective Approaches at Creative Biolabs
We provide a range of tailored methodologies to effectively address our clients' project needs, including but not limited to:
- ELISA
- Flow cytometry
- Surface plasmon resonance / Bio-layer interferometry: real-time, label-free full kinetic analysis.
Service Highlights
- Comprehensive analysis of antibody interaction with FcγRIIa, including kinetics and affinity.
- Offers testing for all major FcγRIIa allelic variants (HH, RR, HR).
- Employs a variety of state-of-the-art technologies for robust data generation.
- Provides customized assay design tailored to specific research objectives.
- Ensures reliable and accurate results through stringent quality control measures.
- Offers expert consultation and dedicated scientific support throughout the project.
Case Study
| Objective: | The study aims to measure the binding affinity of sample-1 with FcγRIIa/CD32a subtypes via the surface plasmon resonance method. | |||||||||||||||||||||||||||||||||
| Assay Format: |
Binding and fitting curves between samples with FcγRIIa/CD32a (R167): steady-state model; Binding and fitting curves between samples with FcγRIIa/CD32a (H167): steady-state model; |
|||||||||||||||||||||||||||||||||
| Results: |
Sample-1 binding to CD32a (R167) We employed the steady-state affinity model to analyze the binding of sample-1 to captured CD32a (R167) (as shown in Fig.2), yielding an affinity constant (KD) of 1.29 × 10-5 M. Sample-1 binding to CD32a (H167) We employed the steady-state affinity model to analyze the binding of sample-1 to captured CD32a (H167) (as shown in Fig.3), yielding an affinity constant (KD) of 4.98×10-6 M. |
|||||||||||||||||||||||||||||||||
|
Table 1 SPR result summary between sample-1 with FcγRIIa/CD32a subtypes. |
||||||||||||||||||||||||||||||||||
Associated Services
Leveraging clinical-grade reference antibodies as positive controls, our experienced scientists at Creative Biolabs excel at customizing FcγRIIa binding assays with optimized parameters to address your unique research questions. To gain deeper mechanistic insight into the in vivo potency of your lead antibodies, we recommend combining this assay with cell-based cytotoxicity assays like ADCC and ADCP, while emphasizing the importance of careful data interpretation due to potential discrepancies with in vivo conditions caused by competing endogenous IgGs. Furthermore, Creative Biolabs offers comprehensive Fc engineering services to modulate antibody Fc characteristics for enhanced interaction with FcγRs precisely.
Customer Review
- Je***e 11/Aug/2024 ⭐⭐⭐⭐⭐
Our preclinical research has benefited much from Creative Biolabs' FcγRIIa binding test service. Accurate and consistent data on the affinity and kinetics of our antibody candidate for both the H131 and R131 alleles gave important new perspectives on its possible effector activities. I valued the team's responsiveness as well as their perceptive direction throughout the process. Their given report was thorough, current, and understandable. Their ability to fit our particular antibody format and the clear information on the experiment settings and findings were appreciated.
For more details about our FcγRIIa binding assay, please contact us immediately.
Reference
- Revelly, Etienne, et al. "How to solve the conundrum of heparin-induced thrombocytopenia during cardiopulmonary bypass." Journal of Clinical Medicine 12.3 (2023): 786. Distributed under Open Access License CC BY 4.0. The original image was modified by extracting and using only part A, and the title was changed to " The FcγRIIa receptor's signaling pathway ".
For Research Use Only.

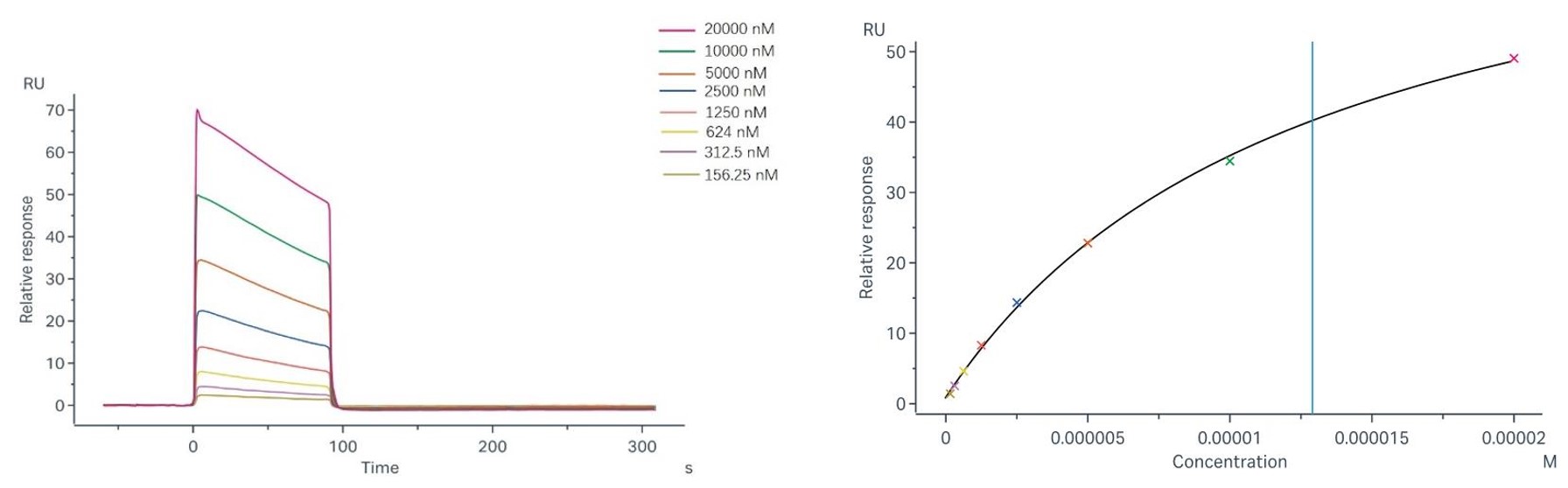 Fig.2 Binding and fitting curves between sample-1 with FcγRIIa/CD32a (R167) at different concentrations.
Fig.2 Binding and fitting curves between sample-1 with FcγRIIa/CD32a (R167) at different concentrations.
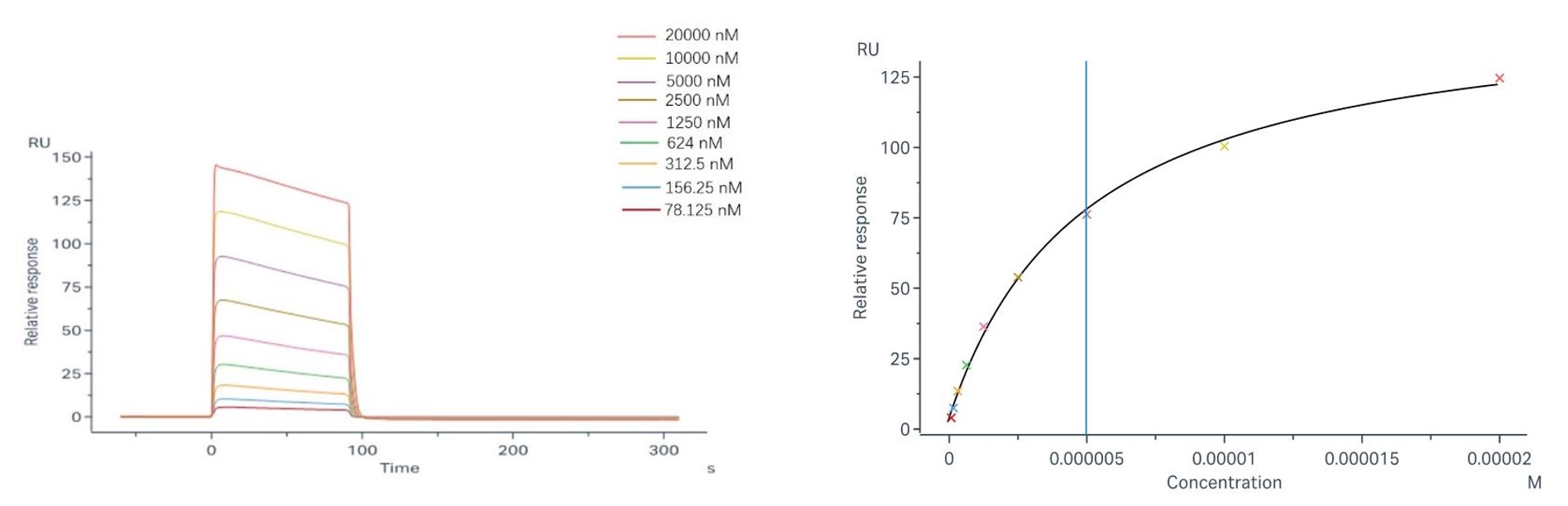 Fig.3 Binding and fitting curves between sample-1 with FcγRIIa/CD32a (H167) at different concentrations.
Fig.3 Binding and fitting curves between sample-1 with FcγRIIa/CD32a (H167) at different concentrations.