Glycerol induced Acute Renal Failure Modeling & Pharmacodynamics Service
Creative Biolabs offers a range of well-established models for evaluating drug efficacy in acute renal failure. These models simulate the physiological conditions of AKI and are valuable tools for testing therapeutic agents aimed at improving kidney function.
Introduction
Acute renal failure (ARF), also known as acute kidney injury (AKI), is a rapid loss of kidney function that occurs over hours to days. It is characterized by a sudden decline in glomerular filtration rate (GFR), resulting in the accumulation of waste products such as urea and creatinine in the blood. ARF can be caused by a variety of factors, including ischemia, nephrotoxic drugs, infections, and systemic diseases. It presents clinically with symptoms such as oliguria, edema, electrolyte imbalances, and metabolic acidosis. The pathophysiology of ARF typically involves acute tubular necrosis, glomerular injury, or interstitial inflammation, leading to kidney dysfunction. If not treated promptly, ARF can progress to chronic kidney disease or even end-stage renal failure, necessitating dialysis or kidney transplantation. Effective treatment strategies for ARF are limited, and early intervention is crucial to prevent long-term kidney damage. Current research focuses on developing therapies to protect the kidneys, restore renal function, and prevent progression to chronic kidney disease. Therefore, the development of reliable animal models to study AKI and evaluate potential treatments is essential.
Glycerol-Induced Acute Renal Failure Model
The glycerol-induced acute renal failure model is established by administering a single dose of glycerol (typically 50% glycerol solution) intramuscularly. This model mimics acute tubular necrosis (ATN) and is widely used for the investigation of kidney injury mechanisms and the evaluation of therapeutic drugs. The key features of this model include rapid onset of renal dysfunction, characterized by elevated blood urea nitrogen (BUN), serum creatinine, and histological signs of tubular injury. The advantage of this model is its simplicity and high reproducibility. It is particularly useful for testing nephroprotective agents and exploring the pathophysiological processes involved in acute kidney injury. However, one limitation is that the model primarily induces injury in the proximal tubules, which may not fully reflect all types of kidney injury seen in humans. Overall, it remains an essential tool for preclinical drug development targeting kidney diseases.
- Simulates: This model simulates acute kidney injury (AKI), specifically focusing on acute tubular necrosis caused by nephrotoxic agents. It is a widely used model to study the mechanisms of kidney damage and repair.
- Evaluates Drugs: The glycerol-induced AKI model is ideal for evaluating potential nephroprotective drugs, anti-inflammatory agents, and compounds aimed at reducing kidney damage. It can also be used for studying the effects of drugs that promote renal recovery and improve renal function post-injury.
Evaluation Platform
- Animals: Rat.
-
Measurements
We offer a variety of measurements to evaluate drug efficacy in the glycerol-induced acute renal failure model, utilizing advanced technologies, including but not limited to:- General observations: Body weight, mortality rate, urine output, and signs of discomfort.
- Biochemical analysis: Blood urea nitrogen (BUN), serum creatinine, and electrolytes.
- Histopathology: Kidney tissue damage (tubular necrosis, edema, and infiltration).
- Immunohistochemistry: Infiltration of immune cells such as neutrophils and macrophages in kidney tissues.
- Cytokine profiling (e.g., ELISA): Levels of inflammatory cytokines such as TNF-α, IL-6, and IL-1β.
- Gene/protein expression profiling: Kidney-specific biomarkers, apoptosis markers, and cell proliferation markers via RT-qPCR and Western blot.
- Urinary biomarkers: Urinary albumin-to-creatinine ratio (ACR) and kidney injury molecule-1 (KIM-1) levels.
Our team also provides expert guidance in experimental design, data analysis, and customized approaches to ensure the most relevant measurements for your research.
Related Services
In addition to the glycerol-induced acute renal failure model, we offer other methods of inducing acute kidney injury, such as cisplatin-induced and ischemia-reperfusion injury models. These alternatives can be customized based on your research objectives.
- Gentamicin-Induced Acute Renal Failure Model
- Cisplatin-Induced Acute Renal Injury Model
- Contrast Agent-Induced Acute Kidney Injury Model
- Folic Acid-Induced Acute Kidney Injury Model
- Lipopolysaccharide (LPS)-Induced Acute Kidney Injury Model
- Cecal Ligation and Puncture (CLP)-Induced Acute Kidney Injury Model
Our advantages
- Expertise in preclinical models: Our team has extensive experience in establishing and optimizing renal failure models.
- Customized approach: We tailor our models and services to meet your specific research needs.
- Comprehensive support: From experimental design to data analysis, we provide end-to-end support.
- High-quality results: Our models are reliable and reproducible, ensuring consistent and meaningful data.
- Advanced technologies: We utilize state-of-the-art equipment and methods for accurate and detailed measurements.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What is the glycerol-induced acute renal failure model used for?
The model is used to study acute kidney injury, specifically acute tubular necrosis, and to evaluate the efficacy of potential therapeutic drugs for kidney protection and repair.
-
2. What types of drugs can be tested using this model?
This model is suitable for testing nephroprotective agents, anti-inflammatory drugs, drugs that promote kidney recovery, and agents aimed at reducing kidney damage.
-
3. How is the model established?
The model is established by administering a single intramuscular dose of glycerol (50% solution) to induce acute renal failure.
-
4. What measurements are taken in the model?
Key measurements include biochemical parameters like BUN and serum creatinine, histopathological analysis, immune cell infiltration, and cytokine levels.
-
5. Can this model be used for chronic kidney injury research?
No, this model is specifically designed to simulate acute kidney injury. Chronic kidney disease models are available for long-term studies.
Published Data
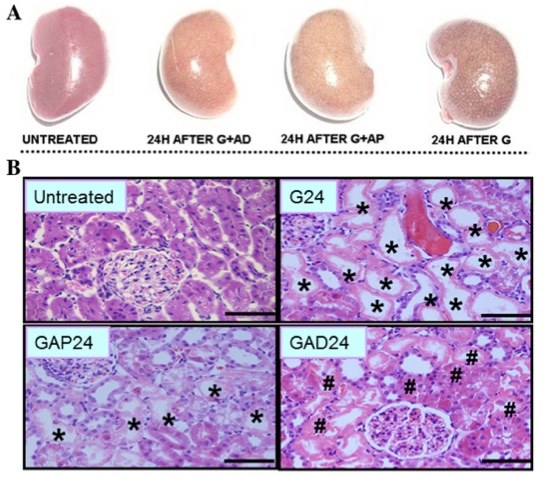 Fig. 1 Effect of anisodamine and atropine on macroscopic and morphological changes.1
Fig. 1 Effect of anisodamine and atropine on macroscopic and morphological changes.1
As shown in Fig. 1a, the kidneys in the untreated group appeared dark pink, indicating healthy organs. In contrast, the glycerol treatment group displayed dark red kidneys with white spots, suggesting organ damage. The kidneys in the anisodamine and atropine treatment groups were light brown and sparsely covered with white spots, indicating injury. Additionally, kidney weight and volume were slightly lower in the anisodamine treatment group compared to the atropine group. Histopathological analysis performed 24 hours after treatment (Fig. 1b) revealed that in the anisodamine and atropine treatment groups, areas of tubular epithelial cell swelling, necrosis, and desquamation were reduced compared to the control group. The anisodamine group exhibited less tissue damage than the atropine group, as evidenced by smaller areas of tubular epithelial necrosis and desquamation.
Reference
- Li, Yun-Feng et al. "Protective effect of anisodamine in rats with glycerol-induced acute kidney injury." BMC Nephrology vol. 20,1 223. 17 Jun. 2019. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1186/s12882-019-1394-y
For Research Use Only.
