In Vivo Fitness Assessment of Drug Candidate
As we all know, molecule properties of drug candidates that allow successful production, purification and formulation do not necessarily mean optimal behavior in vivo, given the complicated in vivo environment of the patients. To investigate the potential problems and evaluate the in vivo fitness during the developability assessment stage, several assays are developed by Creative Biolabs, aiming to get a preliminary understanding of the drug behavior in vivo.
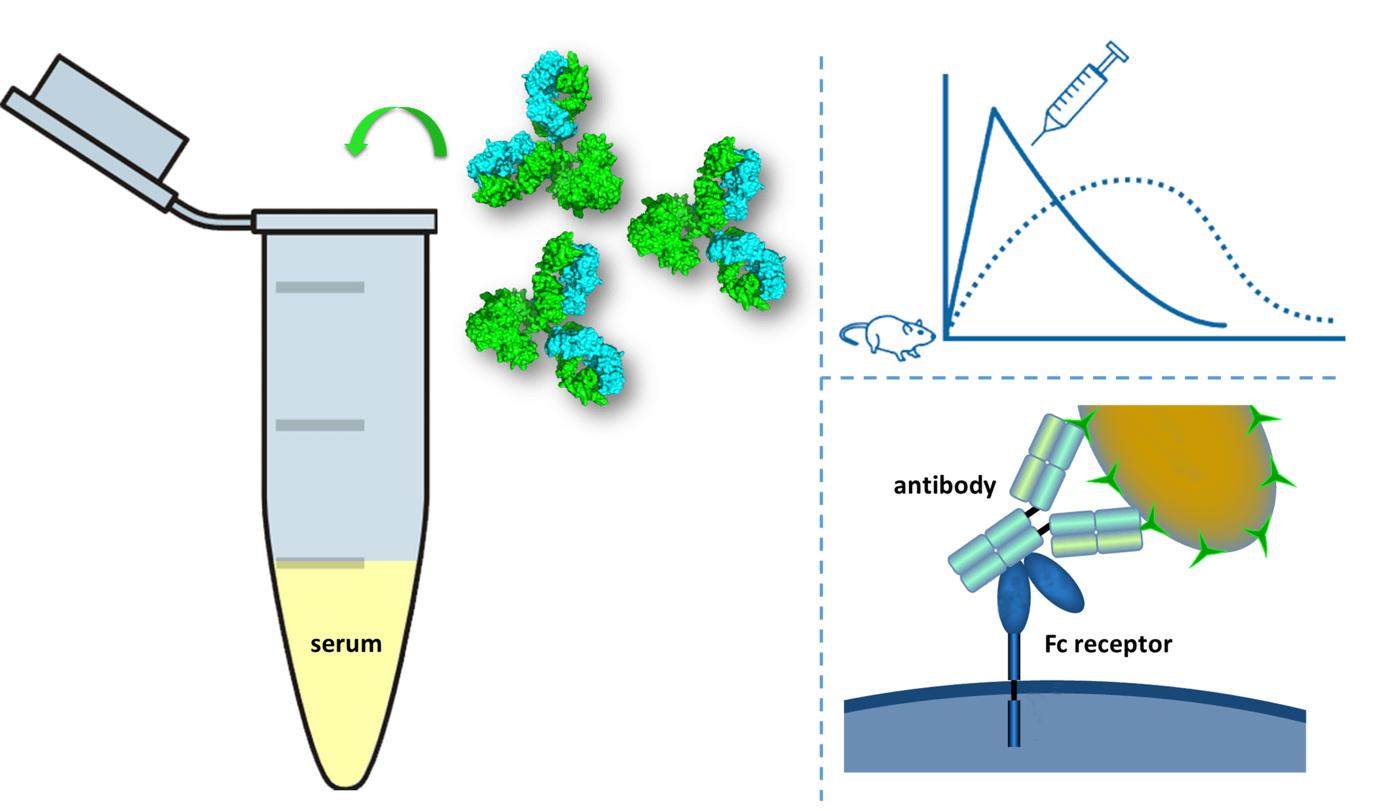 Fig.1 In vivo fitness assessment of drug candidates by the CreDA™ platform.
Fig.1 In vivo fitness assessment of drug candidates by the CreDA™ platform.
Unspecific Binding Assay
Unspecific binding of drug candidates to other proteins is investigated in vitro using the protein microarray technique developed by our developability assessment groups. The objective of this assay is to provide a multitude of protein epitopes for the drug candidates and assess if the binding is restricted to the specifically designed target or not. In short, hundreds of different human proteins are respectively spotted on a nitrocellulose-coated surface, together with the binding target (antigen). In the best case, the tested candidate will only bind to its target. Problematic candidates may show binding affinities to both the target antigen and some of the other spotted proteins, an undesirable result that may influence the candidate ranking in the lead selection.
FcRn Binding Assay
The neonatal Fc receptor (FcRn) helps with the recovery of IgG in adults through the process of endocytosis in endothelial cells: FcRn in acidic endosomes binds to IgG internalized through pinocytosis, recycling it to the cell surface and releasing it at the basic pH of blood, and thereby preventing IgG from undergoing lysosomal degradation. Thus, the ability of IgG to bind to FcRn in an acidic environment and to dissociate at physiological pH conditions is a prerequisite for the long half-life of an antibody drug. Therefore, during the developability assessment, the binding affinities of IgGs to human, cynomolgus monkey, or mouse FcRn are determined at acidic and physiological pH values with a surface plasmon-based assay.
FcγR Binding Assay
Fcγ receptors (FcγR) belong to the immunoglobulin superfamily and are the most important Fc receptors for inducing phagocytosis of opsonized (marked) microbes. For example, macrophages expressing FcγR begin to ingest and kill an IgG-coated pathogen by phagocytosis following engagement of their Fcγ receptors. Another process involving FcγR is antibody-dependent cell-mediated cytotoxicity (ADCC). During ADCC, FcγR receptors on the surface of natural killer (NK) cells stimulate the NK cells to release cytotoxic molecules to kill antibody-covered target cells. Thus, during the early developability assessment of antibody drug, measurement of binding against a selection of cynomolgus or human Fcγ receptors allows predicting the presence or absence of ADCC functionality. These measurements are performed on all IgG formats that are designed to either enhance or silence the ADCC functionality.
Stability in Serum
In circulation, the blood temperature is at 37℃ and antibody therapeutics are exposed for various lengths of time to receptors, as well as enzymes such as mannosidases. Due to these factors, serum has influences on the primary, secondary and higher order structures of antibody drugs. For instance, many post-transcriptional modifications (PTMs) such as asparagine deamidation and methionine oxidation, may occur in the serum, which may result in loss of biological activity and increase of the immunogenicity. Serum stability is assessed ex vivo in cynomolgus monkey and human serum or other relevant matrices to judge compatibility with in vivo conditions. The candidates are incubated at 37°C for several weeks at an appropriate concentration. Samples are typically analyzed via a surface plasmon resonance assay, monitoring both the binding to the target and the integrity of the antibody Fc fragment.
Pharmacokinetics Study
Pharmacokinetics (PK) study in rat with a single intravenous injection is used to determine if the half-life and distribution of the candidate (antibody) match the expected behavior of a human antibody. Antibody concentration is monitored for 28 days using a generic ELISA-like assay. This study is not intended to replace a full PK study but rather aims to judge the compatibility of the candidate with the in vivo environment in an animal. Ideally, the antibody does not cross-react with the rat target to avoid any target-mediated disposition effects. And candidates with abnormal PK profiles will be de-selected.
With a mature drug development pipeline, Creative Biolabs provides a full range of analysis services to characterize the in vivo fitness of your drug candidates. Our scientific team provides customers with help from the experimental design to the establishment and optimization of protocols, collection, and analysis of data, as well as suggestions on the engineering of the candidates. Please contact us for more information and a discussion to see how we can be involved in your projects.
For Research Use Only.
