Intrauterine Growth Retardation (IUGR) Diabetic Modeling & Pharmacodynamics Service
Creative Biolabs provides a wide range of diabetes models to evaluate the efficacy of diabetes-related drugs, helping researchers advance therapeutic solutions for this growing public health concern.
Introduction
Intrauterine Growth Retardation (IUGR) refers to a condition where fetal growth is restricted, often leading to infants being born smaller than expected for their gestational age. IUGR can be caused by various factors, including maternal diseases, poor nutrition, or placental insufficiency. When maternal diabetes, such as Type 1 or Type 2 diabetes, is present, the risk of IUGR increases significantly. In diabetic pregnancies, hyperglycemia can lead to changes in the fetal environment, impairing normal growth and development. IUGR in the context of maternal diabetes is associated with both immediate and long-term health complications. These include metabolic disturbances, such as insulin resistance, obesity, and an increased risk of developing Type 2 diabetes later in life. The offspring of diabetic mothers with IUGR are also at higher risk of cardiovascular diseases and other metabolic disorders in adulthood.
Disease Models and Applications
The Intrauterine Growth Retardation (IUGR)-Diabetic Model is established by subjecting pregnant animals to diabetic conditions, which leads to impaired fetal development and growth restrictions. This model is widely used to mimic the effects of maternal diabetes on offspring, especially regarding metabolic alterations such as insulin resistance and glucose intolerance. The main advantage of this model is its ability to replicate the conditions that result from maternal diabetes, providing insights into early-onset diabetes development. However, the model has limitations in fully simulating the diverse complications of human diabetes, such as neuropathy or retinopathy. Despite these drawbacks, the IUGR-Diabetic model remains a valuable tool for studying the long-term effects of maternal diabetes on offspring health, particularly in metabolic disorders.
- Simulates: The Intrauterine Growth Retardation (IUGR)-Diabetic Model simulates metabolic diseases such as Type 2 diabetes, gestational diabetes, and their effects on offspring development, including insulin resistance, obesity, and glucose intolerance. It also replicates the impact of maternal diabetes on fetal growth and subsequent adult metabolic diseases.
- Evaluates Drugs: This model is effective in evaluating drugs aimed at controlling maternal blood glucose levels, preventing insulin resistance, and managing complications in offspring, such as obesity and metabolic disorders. Additionally, it can be used to assess the efficacy of insulin sensitizers, anti-inflammatory agents, and agents targeting pancreatic beta-cell preservation.
Measurements
We offer a variety of measurements for evaluating drug efficacy in the Intrauterine Growth Retardation (IUGR)-Diabetic Model, utilizing an array of advanced technologies, including but not limited to:
- General observations: body weight, mortality rate, growth retardation, and glucose tolerance in offspring.
- Histopathology analysis: examination of pancreatic tissue for beta-cell morphology and insulin secretion capacity.
- Cytokine profiling (e.g., ELISA): Levels of inflammatory markers such as TNF-α, IL-6, and IL-1β in maternal and fetal tissues.
- Blood glucose monitoring: fasting glucose, glucose tolerance tests, and insulin sensitivity assays in offspring.
- Gene/protein expression profiling via RT-qPCR and Western blot techniques: Expression levels of metabolic and insulin signaling proteins such as GLUT4, IRS1, and PPARγ.
- Hormonal analysis: Insulin and C-peptide levels to assess insulin secretion capacity and resistance.
In addition to the established diabetic disease models, our expertise extends to the development of novel animal models tailored to specific research needs. Our scientific team is available to assist in experimental design, model selection, and data analysis, ensuring a customized and effective approach to your project at every stage.
Related Services
In addition to the Intrauterine Growth Retardation (IUGR)-Diabetic Model, we also offer other diabetic models induced by various methods, such as streptozotocin (STZ) injection, high-fat diet, or genetic manipulation. Each model is tailored to simulate different aspects of diabetes and provide the necessary platform for drug evaluation.
- Non-Obese Type I Diabetes Mouse Model
- Streptozotocin (STZ) induced Type I Diabetes Model
- Alloxan induced Type I Diabetes Model
- db/db Type II Diabetes Mouse Model
- STZ-NA induced Type II Diabetes Rat Model
- Zucker Diabetic Fatty (ZDF) Type II Diabetes Rat Model
- High-Fat Diet & Streptozotocin (STZ) induced Type II Diabetes Model
- Combined Spleen & Partial Pancreas Resection & Glucocorticoid induced Type II Diabetes Model
Advantages
- Expertise: Our team has years of experience in developing and optimizing disease models, particularly in the field of diabetes and metabolic disorders.
- Customization: We offer customized models and services tailored to the unique needs of your research.
- Comprehensive Support: From model development to data analysis, we provide end-to-end support for your project.
- Advanced Technologies: We employ state-of-the-art technologies for precise and accurate measurements, ensuring high-quality data.
- Reproducibility: Our models are carefully validated for reproducibility, ensuring consistent and reliable results.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q: What are the main benefits of using the IUGR-Diabetic Model for research?
A: The IUGR-Diabetic Model is valuable for studying maternal diabetes and its long-term metabolic effects on offspring, including insulin resistance, obesity, and glucose intolerance.
-
Q: How do you assess drug efficacy in this model?
A: We assess drug efficacy using various methods, including general observations, histopathology, cytokine profiling, and blood glucose monitoring.
-
Q: What other diabetic models are available?
A: We offer models induced by STZ, high-fat diet, and genetic manipulation, each providing distinct insights into different aspects of diabetes.
Published Data
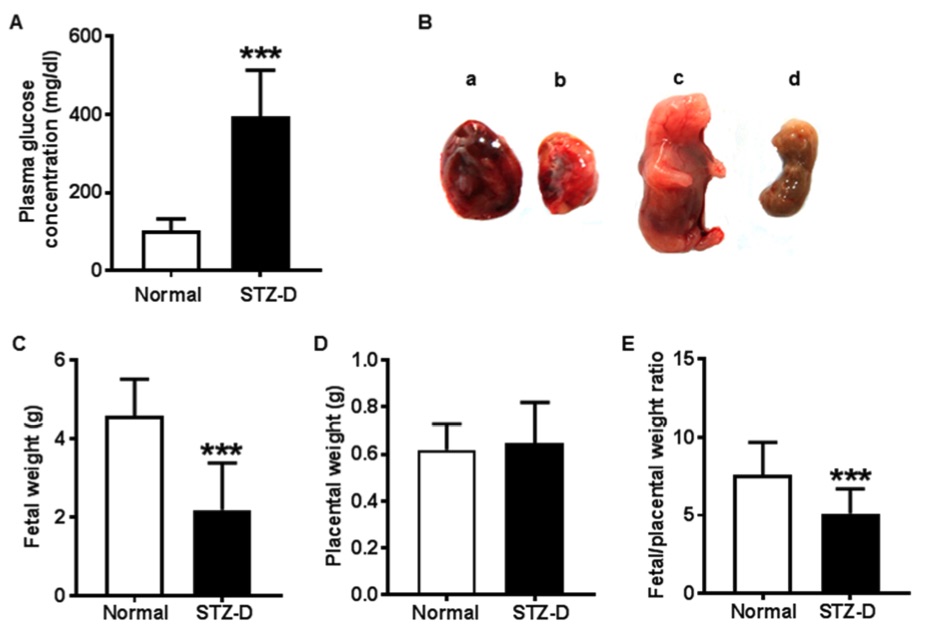 Fig. 1 Pregestational diabetes led to fetal growth restriction and decreased placental efficiency in streptozotocin-induced diabetes (STZ-D) rats.1
Fig. 1 Pregestational diabetes led to fetal growth restriction and decreased placental efficiency in streptozotocin-induced diabetes (STZ-D) rats.1
In this experiment, maternal glucose levels were significantly elevated in rats that were administered streptozotocin (STZ). All rats in the STZ-induced diabetes (STZ-D) group developed severe diabetes, as indicated by blood glucose levels exceeding 300 mg/dl (Figure 1A). The diabetic mothers exhibited typical symptoms of Type 1 diabetes, including polyphagia, polyuria, and polydipsia, although specific data are not shown. Furthermore, maternal diabetes led to negative outcomes for both the placenta and fetuses, as illustrated in Figure 1B. Notably, fetal weight was significantly reduced in the STZ-D group compared to the control group, indicating intrauterine growth restriction in the offspring of severely diabetic mothers (Figure 1C). Interestingly, no significant difference was observed in the placental weight between the groups (Figure 1D). However, the fetal weight-to-placental weight ratio was significantly lower in the STZ-D group, further suggesting compromised fetal development in diabetic conditions (Figure 1E).
Reference
- Xu, Jie et al. "Downregulation of Placental Amino Acid Transporter Expression and mTORC1 Signaling Activity Contributes to Fetal Growth Retardation in Diabetic Rats." International Journal of Molecular Sciences vol. 21,5 1849. 7 Mar. 2020, DOI:10.3390/ijms21051849. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
