LPS induced Pulmonary Neutrophilia Modeling & Pharmacodynamics Service
Pulmonary neutrophilia is a consistent finding in diseases such as human adult respiratory distress syndrome (ARDS), cystic fibrosis (CF), and in chronic obstructive pulmonary disease (COPD). It has been hypothesized that the pulmonary recruitment and activation of neutrophils contribute to the observed dysfunctional symptoms, including increased airway reactivity, the presence of pulmonary fibrosis, and the hypersecretion of mucus. Herein, Creative Biolabs provides LPS-induced mouse and rat models for researchers to investigate the effect of targeting mechanisms related to neutrophil recruitment and mediator release.
Role of Lipopolysaccharide (LPS)
Lipopolysaccharide (LPS) is a key constituent of Gram-negative bacterial cell walls. When delivered to animals and humans, LPS exposure displays major features of microvascular lung injury, including leukocyte accumulation in lung tissue, pulmonary edema, profound lung inflammation, and mortality. As a consequence, LPS-induced animal models highlight ways to explore mechanisms of multiple diseases and provide useful information on the discovery of novel biomarkers and drug targets.
LPS-induced acute pulmonary neutrophilia mice and rat models are characterized by pulmonary neutrophil accumulation and inflammatory mediator production. Thus, they are useful experimental in vivo models for investigating the effect of targeting mechanisms related to neutrophil recruitment and mediator release.
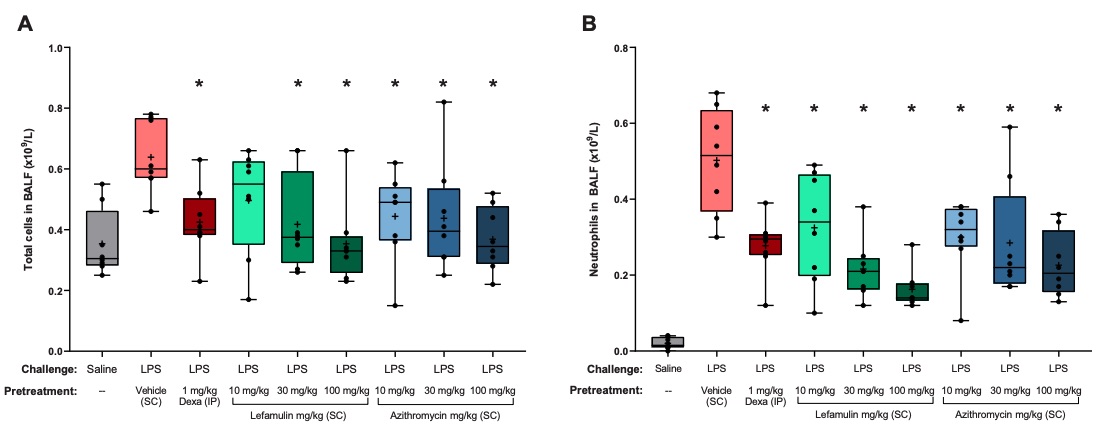 Fig. 1 Total and neutrophil cell counts in BALF of mice with LPS-induced pulmonary neutrophilia. 1
Fig. 1 Total and neutrophil cell counts in BALF of mice with LPS-induced pulmonary neutrophilia. 1
Mouse Models of Pulmonary Neutrophilia
Pulmonary neutrophilia could be induced in mice by intranasal administration of LPS. As a result, pathological changes such as a marked neutrophilia as well as an increased production of inflammatory mediators (e.g., TNF-α) in the bronchoalveolar lavage (BAL) fluid can be observed.
Rat Models of Pulmonary Neutrophilia
For the induction of rat models of pulmonary neutrophilia, either intratracheal (IT) administration of LPS solution or an aerosol of LPS can be used to obtain such models successfully. Genetically susceptible rat species typically include Sprague/Dawley rats and Wistar rats. Similar to mouse models, pathological changes including a marked neutrophilia as well as a significantly increased production of inflammatory mediators in the bronchoalveolar lavage (BAL) fluid can be observed.
Assessments
In testing the therapeutic efficacy of drug candidates, we are capable of conducting all kinds of assessments as well as providing assistance in data interpretation. Our battery of assessments including but not limited to:
- Bronchoalveolar lavage fluid (BALF) analysis
- Determination of cell numbers
- Lung histology examination
- Measurements of inflammatory chemokines and cytokines
- Immunohistochemistry
Additionally, Creative Biolabs is staffed with experienced personnel and is equipped with essential equipment to generate a wide range of disease models. Therefore, apart from the model described above, Creative Biolabs offers the following other rodent respiratory disease models:
- Ovalbumin-Induced Asthma Model
- House Dust Mite (HDM)-Induced Allergic Asthma Model
- Cigarette Smoke-Induced COPD Model
- Bleomycin-Induced Lung Fibrosis
Here at Creative Biolabs, an extensive array of services can be provided to meet every specific requirement of our clients in the early drug discovery and development phase. Moreover, new animal models can be tailored to your specific research purposes and to guarantee that any data collected is both biologically and clinically reliable and relevant. If you're interested in our services, contact us to discuss your requirements.
Reference
- Hafner, Michael, et al. "Anti-inflammatory activity of lefamulin versus azithromycin and dexamethasone in vivo and in vitro in a lipopolysaccharide-induced lung neutrophilia mouse model." PLoS One 16.9 (2021): e0237659. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
