LPS induced Sepsis Modeling & Pharmacodynamics Service
As one of the most reliable and well-recognized research partners, Creative Biolabs offers diverse rodent models for preclinical research of anti-septicemic drug candidates, including CLP sepsis model, LPS-induced sepsis model as well as new sepsis model development services.
Animal Models in Sepsis Research
As a life-threatening complication, sepsis arises when the body's response to infection causes injury to its own tissues and organs. The disease is accompanied by systemic inflammation with excessively produced pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). Animal models that have been developed to mimic human sepsis aiming to study the pathogenesis as well as to test potential therapeutic agents are diverse in sepsis research. Specifically, sepsis animal models fall into three different classes: injection of an exogenous toxin, alteration of the animal's endogenous protective barrier, and instillation of exogenous bacteria.
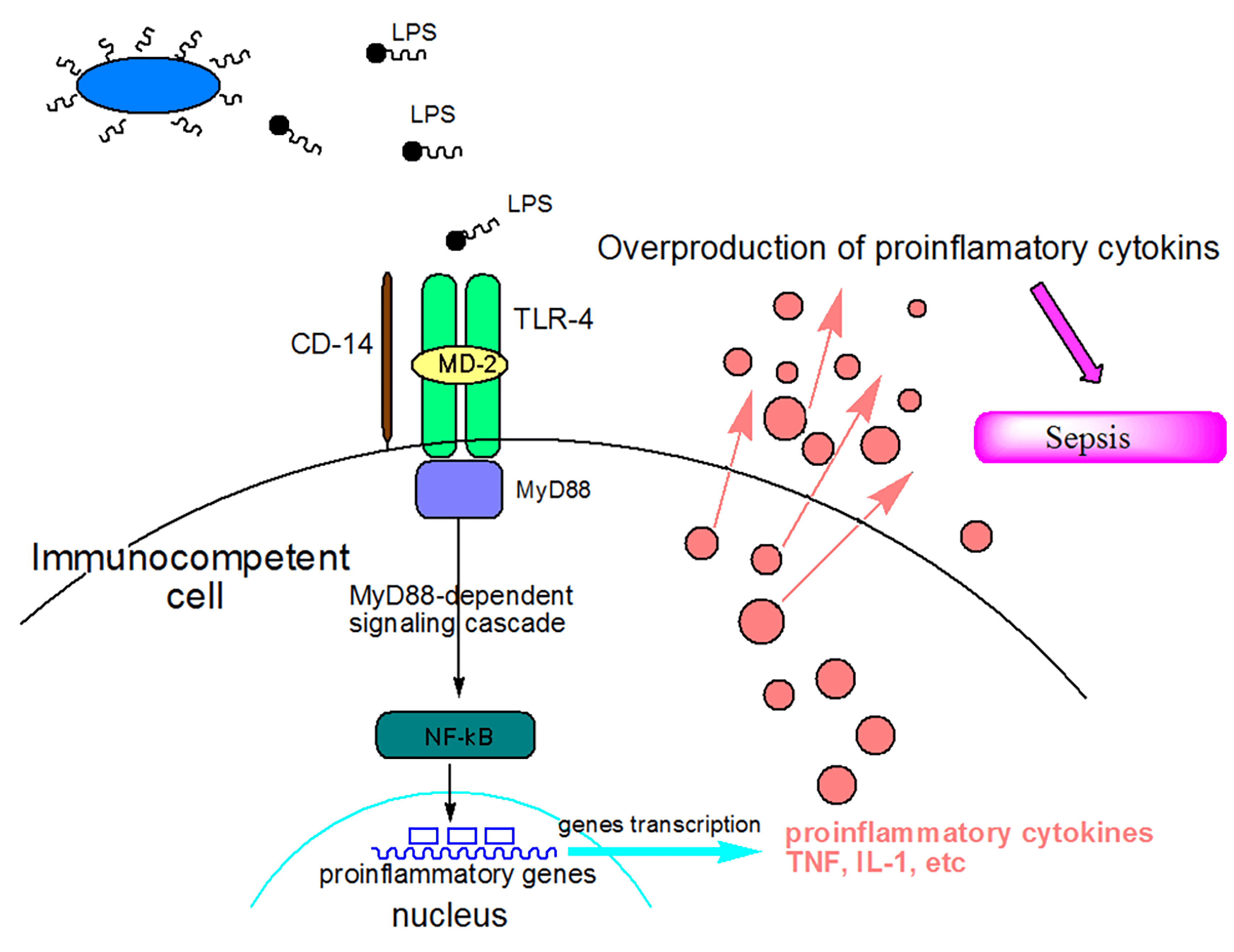 Fig.1 Mechanisms of action of lipopolysaccharide (LPS) as a sepsis inducer.
Fig.1 Mechanisms of action of lipopolysaccharide (LPS) as a sepsis inducer.(Tamara et al. 2010)
Overview of LPS-Induced Sepsis Rodent Models
Endotoxin (LPS), the outer membrane component of Gram-negative bacteria, can elicit strong immune responses in animals and thereby is widely applied as a pathogenic factor in sepsis research. LPS administration induces systemic inflammation that mimics many of the initial clinical features of sepsis, including extensive proinflammatory cytokine production such as TNF-α and IL-1, which leads to multiple organ failure and a high lethality rate. Toll-like receptor (TLR-4) is the receptor for lipopolysaccharide (LPS). Binding of LPS to TLR-4 activates the downstream signaling pathway, including mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) pathways in macrophages, which play a vital role in regulating the secretion of pro-inflammatory cytokines and mediators.
Pros and Cons of LPS-Induced Sepsis Rodent Models
LPS injection is a simple and sterile way to induce sepsis and it shares many similarities with the initial phase of human sepsis. However, LPS-induced sepsis models show weakness in preclinical research. Firstly, sepsis caused by Gram-negative bacterial infection displays a very rapid and transient increase in systemic cytokine levels, while human sepsis is characterized by prolonged elevation and the level is several orders of magnitude lower. Also, such symptoms of LPS infusion as renal hypoperfusion and increased blood urea nitrogen are alleviated by volume replacement, which is routinely performed in clinical management of sepsis. Nevertheless, this model has been used for studying systemic and renal responses during the initial phases of sepsis.
The Induction of Sepsis in Rodents
Drug candidates are intragastrically (or intraperitoneally) administered in advance, after which the animals receive LPS in sterile PBS by intraperitoneal injection and blood is collected afterward. Cytokines, such as TNF-α and IL-6, are measured at various time points and the survival states are recorded. Optional assessments provided by Creative Biolabs including but not limited to:
- Clinical Observations
- PK/PD Blood Collections
- Biochemistry Analysis
- Body Weight Measurement
- Analysis of Macrophages
- Histopathology
- Immunohistochemistry
Meanwhile, in order to meet our customers' specific requirements and their various research objectives, Creative Biolabs also offers other rodent inflammatory & immunological disease models listed as follows that you may be interested in:
- Carrageenan Air Pouch Model
- Carrageenan-Induced Paw Edema Model
- Imiquimod (IMQ)-Induced Psoriasis Rodent Model
- Chemical-Induced Rodent Contact Hypersensitivity Model
- Passive Cutaneous Anaphylaxis (PCA) Model
- Delayed Type Hypersensitivity (DTH) Rodent Model
- Adjuvant-Induced Arthritis (AIA) Rodent Model
- Collagen-Induced Arthritis (CIA) Rodent Model
- Collagen Antibody-Induced Arthritis (CAIA) Model
- Cecum Ligation and Puncture (CLP)-Induced Sepsis Model
- Spontaneous Systemic Lupus Erythematosus (SLE) Mouse Models
- Induced Models of Systemic Lupus Erythematosus
Creative Biolabs is happy to share our cutting-edge technology and extensive expertise in rodent disease models to facilitate our clients' research and project development. Please feel free to inquire us for more detailed information if you are interested in any of these services. If you have a specific need, then contact us to discuss your requirements.
Reference
- Tamara, S.;et al. Marine compounds with therapeutic potential in Gram-negative sepsis[J]. Marine Drugs. 2013, 11(6):2216-2229.
For Research Use Only.
