Hi-Affi™ Humanized ICOSL Immune Checkpoint Knockin Mouse Model
The discoveries of immune checkpoints are breakthrough for human cancer treatment and immune-oncology-related therapies have been either approved or on trial. Inhibition of immune checkpoints enhances the anti-tumor immunity and exhibits favorable efficacy in cancer treatment. Up to now, several immune checkpoint inhibitors have been approved for clinical therapy. ICOSL, a signaling promoting the function of regulatory T cells (Tregs), is recognized as a novel target in cancer treatment. Creative Biolabs has successfully established an optimized Hi-Affi™ “humanized” animal platform to offer specialty manipulated ICSOL immune checkpoint knock-in mice for our clients all over the world.
ICOSL Molecule
Upon the discovery of immune checkpoints, plenty of studies have investigated their activities in the anti-tumor action of host immune system. The host immune system could recognize tumor cells following the inhibition of the immune checkpoints. Subsequently, the cytotoxic T cells are activated to kill the tumor cells in cancer patients. Thus, targeting the immune checkpoints using small molecules or monoclonal antibodies have become the “hot zone” in tumor immunotherapy.
ICOSL (also known as b7-associated protein-1), belongs to B7 superfamily and is a ligand of ICOS. ICOSL residents in dendritic cells (DCs), B lymphocytes, and cancer cells including melanoma, breast cancer, glioma cells, and hematologic neoplasm cells. The interaction of ICOSL with its unique receptor ‘ICOS’ triggers activation of T cells, production of cytokines, and downstream differentiation into Treg cells which could influence immune-mediated cancer tolerance. Thus, ICOSL begins to draw attention to its potential usage in cancer treatment.
ICOSL Immune Checkpoint Pathway
The ligation of ICOSL and ICOS mediates the function of Tregs infiltrating in the tumor, resulting in immune evasion in various cancer such as melanoma and breast cancer. Specifically, ICOS/ICOSL axis could promote the activation and expansion of Tregs. Besides this promotion, ICOS/ICOSL could enhance the IL-10 production of Tregs. Recent studies found that the ICOSL expression level was significantly higher in the cancerous tissues when compared to non-cancerous tissues in breast cancer. The blockade of the ICOS/ICOSL pathway has been shown to exhibit a negative modulatory effect on Tregs and T helper 2 (Th2) cells, thus potentially protecting against cancer growth.
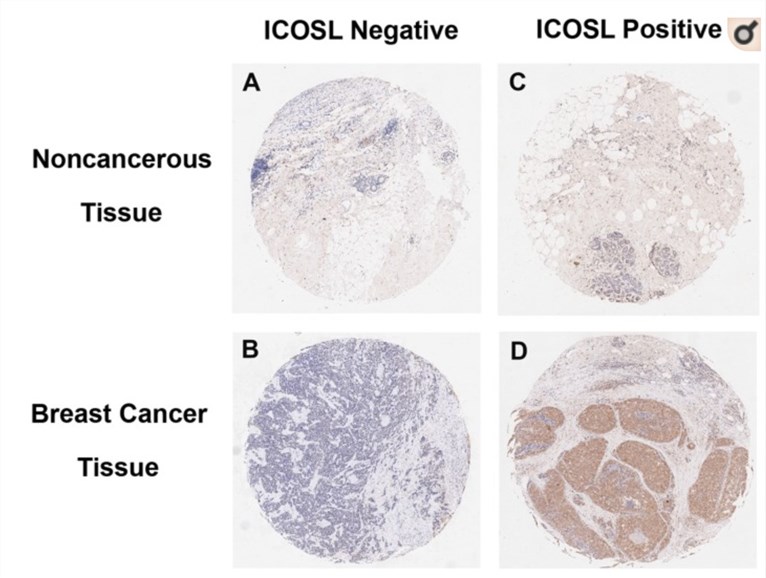 Fig.1 Images of breast cancer immunostaining for ICOSL in cancerous and noncancerous tissues. (Bin, 2019)1, 2
Fig.1 Images of breast cancer immunostaining for ICOSL in cancerous and noncancerous tissues. (Bin, 2019)1, 2
Development of Humanized ICOSL Immune Checkpoint Knock-In Mice
Blockade the immune checkpoints can eliminate the immunosuppression and mobilize the host immune system to fight against the tumor, which has been believed as an effective and powerful strategy in cancer treatment. As a novel immune checkpoint, ICOSL has been evidenced its role in immunosuppression. Blockade the ICOSL has potential in cancer treatment and anti-ICOSL antibodies have been widely investigated in tumor models. With advanced technology, Creative Biolabs has successfully established an array of well-established Hi-Affi™ “humanized” animal models, especially for ICOSL immune checkpoint knock-in mice. Our scientists focused on humanized mouse models are pleased to assist you in your studies of anti-tumor immunotherapies. What’s more, we also established a comprehensive in vitro immunomodulation assessment service using various approaches. Please feel free to contact us or more details.
Creative Biolabs also offers other various Humanized Mouse Models you may be interested in:
References
- Bin, W.; et al. Expression of ICOSL is associated with decreased survival in invasive breast cancer. PeerJ. 2019, 7:p.e6903.
- under Open Access license CC BY 4.0, without modification.
For Research Use Only.
