Ophthalmic Toxicity
Currently, Creative Biolabs offers this unique opportunity for our global clients to adopt integrated ophthalmic toxicity testing service using our state-of-the-art technique systems.
The ophthalmic system is sensitive and venerable which is constantly exposing to outside environmental conditions and substances on a daily basis. Many drug components can cause ophthalmic damages ranging from mild irritation and inflammation to tissue corrosion leading to irreversible blindness. For example, ethambutol, one routine therapy for tuberculosis treatment, has been proven to induce severe ocular toxic side effects, i.e. retrobulbar neuritis and even permanent visual disability. Hence, ophthalmic toxicity assessment is required by regulatory agencies to ensure that eye-associated risks meet acceptable safety criteria and are clearly labeled for the appropriate application.
During early years live animals were widely employed to assess ophthalmic hazard. The rabbit in vivo eye test (Draize test) had been performed as the international standard assay for acute ocular toxicity since the 1940s. However, as testing techniques flourish and ethical reconsideration of animal use in toxicity studies arises, Draize test along with other animal toxicity tests are gradually replaced by advanced ex vivo and in vitro approaches. With vast expertise after years of devotion, Creative Biolabs has developed multiple well-recognized in vitro assays for evaluating ophthalmic risks of lead compounds. We can characterize ocular effects of compounds by different administration routes (including topical, oral, etc.) in various forms (including liquids, solids, semi-solids, gels, etc.)
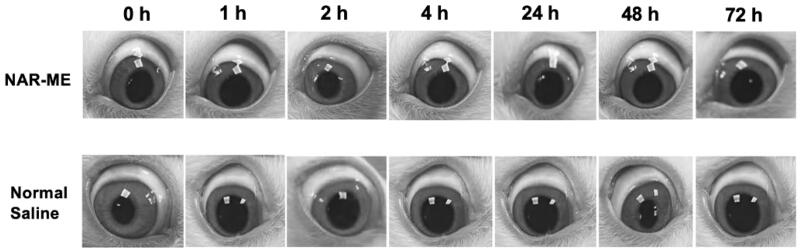 Fig.1 Local ocular reaction observed under slit lamp microscope after in vivo single instillation of 0.5% NAR-ME or normal saline (served as control).1
Fig.1 Local ocular reaction observed under slit lamp microscope after in vivo single instillation of 0.5% NAR-ME or normal saline (served as control).1
Ocular Irritation Test
Creative Biolabs takes advantage of the exclusive in vitro 3D EpiOcular™ model. By culturing human-derived epidermal keratinocytes on designed scaffolds, this model can form stratified, squamous epitheliums that closely resembles the human native ocular tissue. This highly organized model achieves 96% sensitivity with specificity of 63% and accuracy of 80% compared to Draize test and is approved for ophthalmic toxicity detection by the regulatory department.
After exposed to indicate test compounds, alterations in the model tissue will be determined by multiple endpoints, such as MTT assay, FL assay, and enzymatic assessments. We can conduct tailored protocols with different concentrations, time points, and exposure methods.
Short Time Exposure (STE) for Severe Eye Damage
Since short time exposure is believed to be more similar to actual exposure conditions, STE is widely used for severe ophthalmic hazard classification and labeling of chemical substances. After treating a confluent monolayer of rabbit corneal cells with test compounds for a relatively short period (5 min), the toxic outcomes will be estimated by MTT test. This fast and high throughput test is recommended for serious irritants detection (i.e. UN Globally Harmonized System of Classification and Labelling of Chemicals (GHS) Category 1) with no further testing, or identification of risk-free ocular chemicals (i.e. UN GHS Category No) in a tiered strategy.
Equipped with various elegant models as well as sophisticated assessing techniques, Creative Biolabs is confident in bringing consistent, in-depth ophthalmic toxicity profiles to our clients. We can customize our testing process with varied endpoints, time points, and exposure methods to address specific issues. As an alternative, Creative Biolabs also offers human ocular primary cell lines for biological assays, such as Western blotting and immunocytochemistry, for better evaluate ophthalmic toxicity potential. For more detailed information, please feel free to contact us or directly sent us an inquiry.
Reference
- Yu Ma, et al. "Development of a naringenin microemulsion as a prospective ophthalmic delivery system for the treatment of corneal neovascularization: in vitro and in vivo evaluation." DRUG DELIVERY 29.1 (2022): 111–127. under Open Access license CC BY 4.0, without modification.
For Research Use Only.
