Pharmacokinetic/Toxicokinetic (PK/TK) Analysis
During the past years, Creative Biolabs has gained a wealth of good reputation for successfully providing numerous ADA immunogenicity evaluation services. Now we are happy to introduce our ADA pharmacokinetic/toxicokinetic (PK/TK) analysis services for worldwide customers.
Introduction of ADA Pharmacokinetic/Toxicokinetic (PK/TK) Analysis
Administration of biological drugs such as humanized, fully human monoclonal antibodies or recombinant proteins can induce the formation of anti-therapeutic antibodies, commonly known as anti-drug antibodies (ADAs) in both preclinical animals and clinical subjects. The formation of ADA often elicits an immunological response that may or may not neutralize the target binding of the drug, alter the clearance rate, and interfere in the PK assay used to measure the drug concentration in circulation. Thus, ADA can significantly confound the quantitation of drug levels in pharmacokinetic/toxicokinetic (PK/TK) analysis and may result in insufficient exposure to calculate safety margins from toxicology studies. In this case, the analysis of PK/TK changes caused by ADA is a critical part of preclinical drug development.
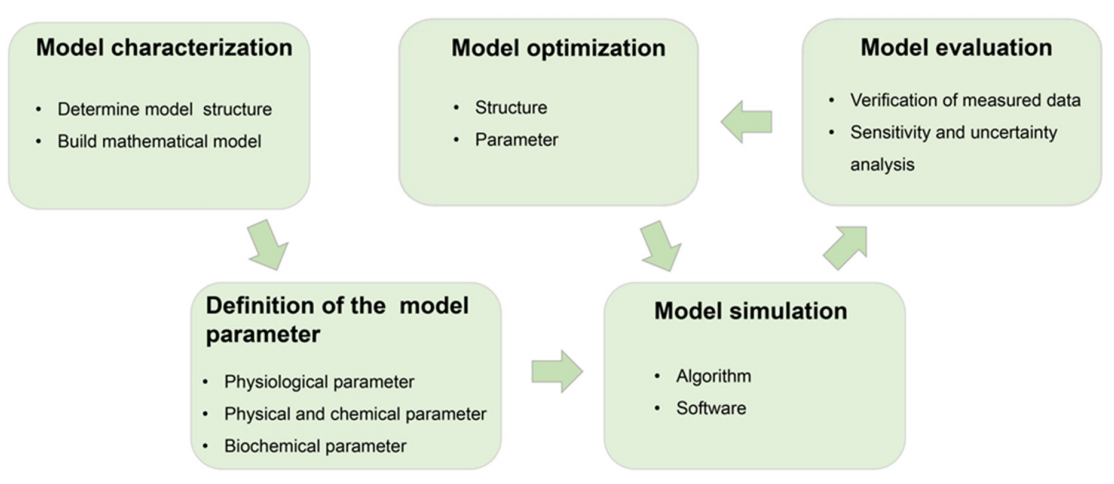 Fig.1 The diagram for the construction of the PBTK model.1, 2
Fig.1 The diagram for the construction of the PBTK model.1, 2
ADA Pharmacokinetic/Toxicokinetic (PK/TK) Analysis Service Provided by Creative Biolabs
ADA can cause perturbation of pharmacokinetic profiles, making it difficult to interpret PK/TK data, particularly when target-mediated drug disposition (TMDD) is suspected. The simplest way to determine the cause of abnormal PK when both ADA and TMDD are the suspects is to measure ADA levels in the serum or plasma of the dosed animals. With years of experience and professional scientists, Creative Biolabs is capable of designing and conducting in vivo PK studies in the appearance of ADAs in support of biological drug IND enabling. We are able to provide a variety of assay formats for the detection of ADA and tested article levels, including but not limited to radioimmunoassays, LC-MS/MS, HPLC, CE or immunochemistry. Moreover, toxicokinetic assessments can be conducted in parallel or concurrent with ongoing toxicology studies.
Features of Our Services
- Multiple approaches for ADA detection
- Fast turnaround time and low cost
- High-quality data for regulatory submission
- Professional technical support team
Based on advanced technology platform, Creative Biolabs is committed to providing high-quality ADA immunogenicity evaluation services for global customers. In addition to ADA pharmacokinetic/toxicokinetic (PK/TK) analysis service, we also provide other immunogenicity assessment services for global customers. We are confident to promote your project a success. If you are interested in the services we provide, please feel free to contact us for more information.
References
- Chen, Mengting, et al. "The Application of a Physiologically Based Toxicokinetic Model in Health Risk Assessment." Toxics 11.10 (2023): 874.
- Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
