Reaction Condition Optimization
As new drugs are on the market constantly, the strategy to improve drug development and manufacturing processes becomes critical. In the development of pharmaceutical chemicals, it is increasingly important to improve product yields, reduce waste and cost by optimizing the reaction condition. At the same time, optimizing the reaction condition is part of a strategy to bring new chemical entities to market or extend existing product life cycles.
Creative Biolabs provides reaction condition optimization service by experts with a wealth of knowledge and experience. And the reaction depends on various factors: the concentration of the reactants, the temperature, the physical state and surface area of the reactants, and the nature of the solvent. By optimizing these reaction conditions, chemists can achieve the desired result.
- Concentration: Two substances cannot react with each other unless their constituent particles (molecules, atoms or ions) are in contact. Without contact, the reaction rate will be zero. In contrast, the more reactant particles collide per unit of time, the more frequent their reaction occurs. Therefore, the reaction rate generally increases with increasing reactant concentration.
- Temperature: Increasing the temperature of the system increases the average kinetic energy of its constituent particles. As the average kinetic energy increases, the particles move faster, so they collide more frequently per unit time and possess greater energy, which could increase the speed of response. Therefore, the reaction rate of almost all reactions increases with increasing temperature. In contrast, the reaction rate of almost all the reactions decreases with decreasing temperature. For example, refrigeration could reduce the rate of growth of bacteria in the food. At the meantime, in systems with more than one reaction, the same reactants can produce different products at different reaction temperature. For example, ethanol is converted to diethyl ether in the presence of dilute sulfuric acid at a temperature of about 100℃; however, at 180 ℃ a completely different reaction takes place with ethylene as the main product ( Fig.1).
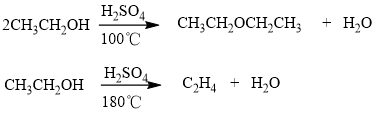 Fig.1 Ethanol produces different products at different reaction temperature.
Fig.1 Ethanol produces different products at different reaction temperature.
- Phase and surface area: If the reactants are evenly dispersed in a single homogeneous solution, the number of collisions per unit time depends on the concentration and the temperature. However, if the reaction is heterogeneous, the reactants are in two distinct stages and collisions between the reactants can only occur at the interface between the stages. The number of collisions between reactants per unit time is greatly reduced with respect to the homogeneous phase, so the rate of reaction is the same. For example, automotive engines use surface area effects to increase reaction rates. Gasoline is injected into each cylinder and burned in the cylinder by gasoline spark ignition. Gasoline injected in the form of microscopic droplets burns more rapidly than fuel that is supplied to the cylinder in a gas stream as it has a larger surface area in that form.
- Solvent: The nature of the solvent also affects the reaction rate. For example, sodium acetate solution and methyl iodide react in an exchange reaction to form methyl acetate and sodium iodide. The reaction is 10 million times faster in the organic solvent dimethylformamide (DMF) than in methanol (CH3OH), because methanol can form hydrogen bonds with acetate ions, whereas DMF cannot (Fig.2), and hydrogen bonding would reduce the reactivity of oxygen atoms in acetate ions.
Creative Biolabs is proud to provide you reaction condition optimization service. Our experts have been developing and implementing a wide variety of pathways in chemical reactions. Please feel comfortable to contact us if you want to know more about reaction condition optimization.
For Research Use Only.
