Based on your target and therapeutic agent, we select the most appropriate RO assay platform (e.g., flow cytometry, radioligand binding, immunohistochemistry). This step involves optimizing critical parameters such as cell density, incubation times, temperature, and reagent concentrations to ensure assay sensitivity and specificity. The expected outcome is a validated, robust assay protocol tailored to your project.
Receptor Occupancy (RO) Assay
The Critical Role of Receptor Occupancy Assays in Drug Development
In the dynamic landscape of drug discovery and development, understanding the precise interaction between a therapeutic agent and its target is paramount. Receptor Occupancy (RO) Assays provide quantitative insights into this critical binding, directly measuring the proportion of target receptors bound by a drug. This information is indispensable for establishing pharmacokinetic/pharmacodynamic (PK/PD) relationships, guiding optimal dose selection, and predicting clinical efficacy. In the evolving landscape of gene therapy, where novel therapeutic targets emerge from genetic modifications, understanding the precise engagement of therapeutic agents with these targets is paramount. RO assays provide the quantitative insights necessary to optimize dosing and predict efficacy for these cutting-edge modalities, reducing costly late-stage failures and accelerating the delivery of life-changing treatments.
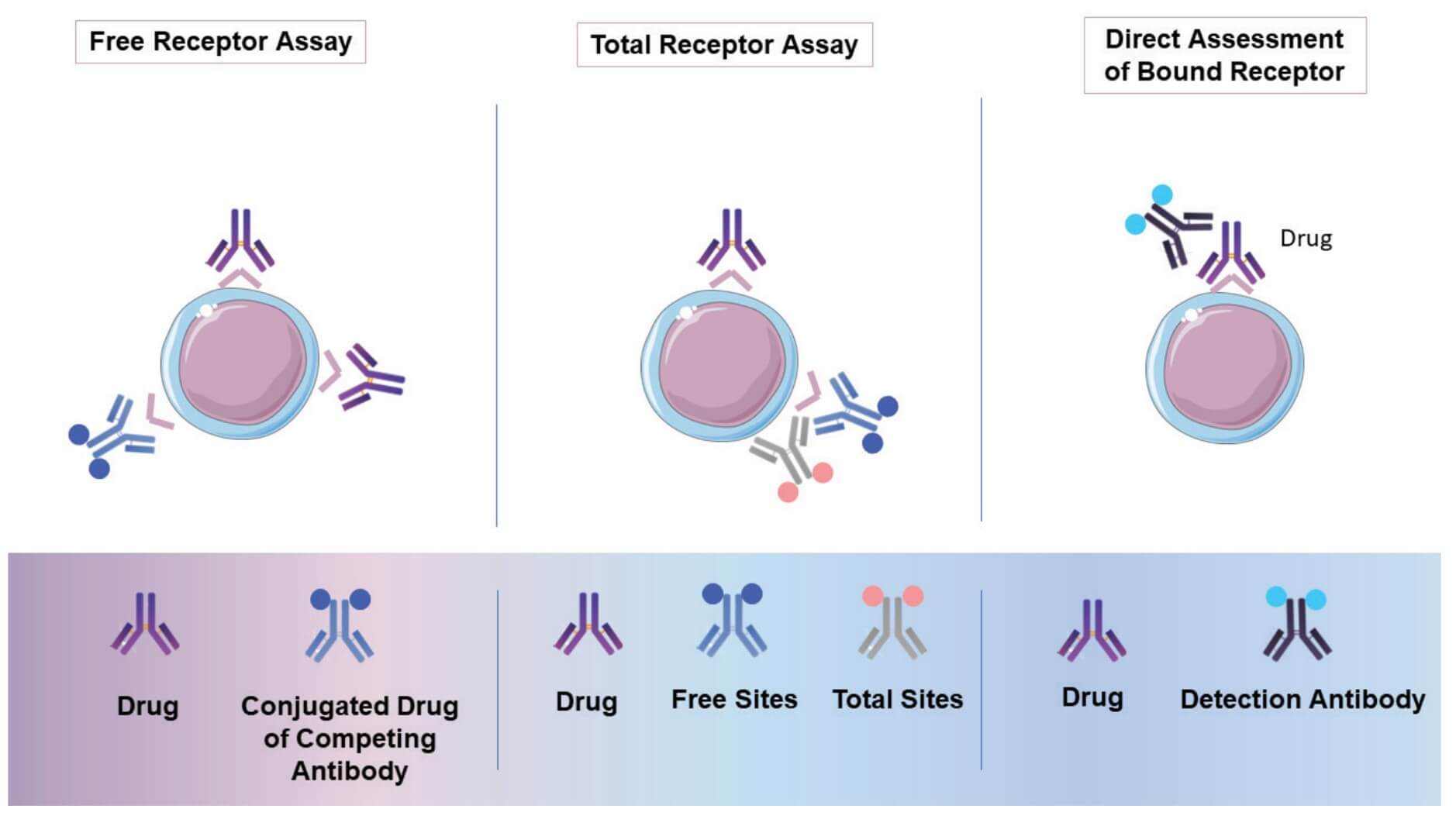 Fig.1 Three RO assay formats.1,3
Fig.1 Three RO assay formats.1,3
Receptor Occupancy (RO) Assays at Creative Biolabs
Creative Biolabs offers comprehensive Receptor Occupancy (RO) Assay services designed to provide crucial data for your drug development pipeline. Utilizing advanced methodologies such as flow cytometry, radioligand binding, and imaging techniques, we meticulously prepare and analyze your samples to precisely quantify drug-target engagement. Our solutions deliver robust quantitative data, including precise receptor occupancy percentages and dose-response profiles, enabling informed decisions from preclinical development through clinical trials. You can expect clear interpretation and actionable insights to advance your therapeutic candidates, with typical project timeframes ranging from 4 to 12 weeks, depending on complexity.
Required Starting Materials
- Target Information: Detailed characterization of the target receptor (e.g., sequence, expression system, known binding partners, cellular localization).
- Therapeutic Agent: Sufficient quantities of the drug candidate (e.g., small molecule, antibody, biologic) with known purity and concentration.
- Relevant Cell Lines/Tissues: Specific cell lines expressing the target, or tissue samples from animal models/clinical biopsies.
Our Service Workflow: From Concept to Clinical
Assay Design and Optimization
Sample Preparation and Treatment
Cells or tissue samples are prepared according to the optimized protocol. This may involve culturing cells, isolating primary cells, or processing tissue biopsies. Samples are then treated with varying concentrations of the therapeutic agent to achieve different levels of receptor occupancy. The outcome is a set of carefully prepared samples ready for binding analysis.
Ligand Binding and Detection
A labeled probe (e.g., fluorescently tagged antibody, radiolabeled ligand) is introduced to quantify unbound receptors. For flow cytometry, cells are stained and analyzed. For radioligand binding, radioactivity is measured. For immunohistochemistry, stained tissue sections are imaged. This step yields raw data reflecting the level of bound and unbound receptors.
Data Acquisition and Analysis
Raw data from the detection step are collected using specialized equipment (e.g., flow cytometer, gamma counter, imaging system). The data are then analyzed using advanced software to calculate receptor occupancy percentages across different drug concentrations. This stage produces quantitative RO curves and binding parameters.
Interpretation and Reporting
Our expert scientists interpret the generated RO data in the context of your drug development goals. This includes correlating RO with drug concentration, assessing target engagement, and providing insights into optimal dosing strategies. You receive a comprehensive report detailing the methodology, raw data, analyzed results, and expert conclusions.
RO Assay Formats
These are three basic formats that have been used as PD measurements in drug development.
| Free Receptor Assay | Drug-Occupied Receptor Assay | Total Receptor Assay |
|---|---|---|
| This format quantifies the available, unoccupied receptors on the cell surface. It is crucial for determining the therapeutic window and potential saturation of a drug target, particularly for antibody-based therapies. By measuring the remaining unbound receptors, it provides direct insight into the drug's ability to engage its target effectively. | This assay directly measures the proportion of receptors that are bound by the therapeutic agent. Utilizing a detection reagent that specifically recognizes the drug when it is bound to the receptor, this format offers a direct assessment of drug-target engagement, essential for understanding pharmacodynamic effects and optimizing dosing strategies. | This format determines the total number of receptors expressed on the cell surface, irrespective of whether they are free or occupied by a drug. It serves as a vital control to ensure that observed changes in free or occupied receptors are due to drug binding and not variations in receptor expression levels, providing a comprehensive understanding of receptor dynamics. |
Service Highlights
- Highly Accurate and Quantitative Data: Our services provide precise measurements, enabling accurate dose selection.
- Reduced Clinical Trial Risks: Quantitative data from RO assays helps in making informed decisions, minimizing uncertainties in clinical development.
- High Sensitivity: Our assays are designed to detect even subtle changes in receptor occupancy, providing detailed insights.
- Broad Platform Compatibility: We utilize various advanced methodologies, ensuring the most suitable approach for your specific target and therapeutic agent.
- Deep Expertise: Benefit from our extensive scientific knowledge and experience in drug-target engagement.
- Streamlined Processes: Our efficient workflows accelerate your project timelines, saving valuable time and resources.
- Faster Time to Market: By providing critical data for early decision-making, we help bring your therapeutic candidates to market more quickly.
Published Data
A study investigated two distinct flow cytometry methods for quantifying CCR5 receptor occupancy (RO) using an anti-CCR5 antibody. The findings demonstrated that both methodologies yielded comparable CCR5 RO values, exhibited minimal background signal in untreated CCR5+CD4+ T cells, and allowed for sensitive occupancy assessments on both circulating and tissue-resident CD4+ T cells. Furthermore, these measurements showed a temporal correlation with plasma concentrations in macaques treated with the anti-CCR5 antibody.
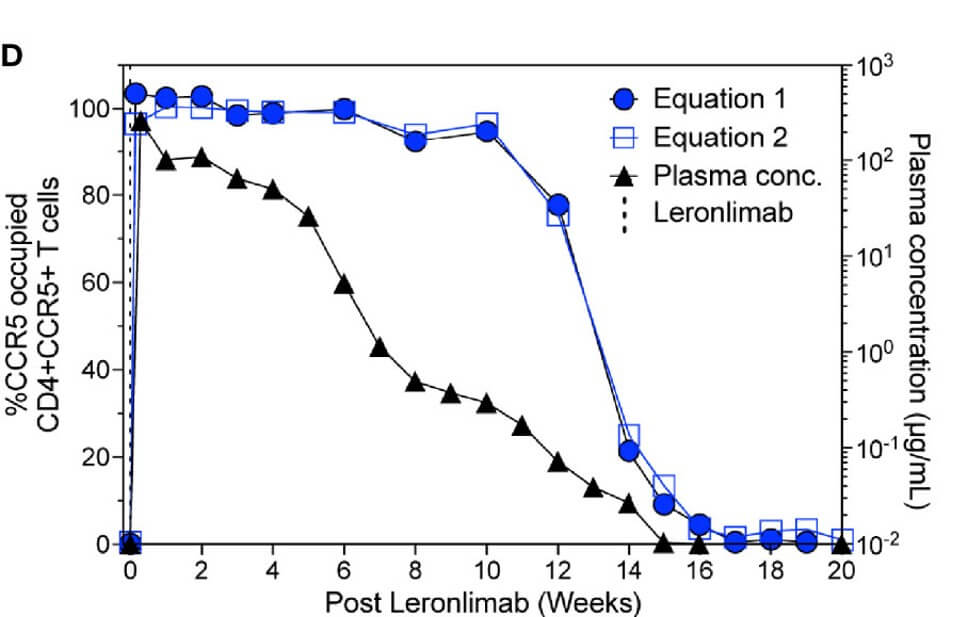 Fig.2 Assay for CCR5 receptor occupancy.2,4
Fig.2 Assay for CCR5 receptor occupancy.2,4
Frequently Asked Questions
-
Q1: What types of receptors can Creative Biolabs' RO assays quantify?
A1: Our Receptor Occupancy Assays are highly versatile and can quantify occupancy for a wide range of targets, including G protein-coupled receptors (GPCRs), receptor tyrosine kinases (RTKs), ion channels, and various surface receptors. We encourage you to discuss your specific target with our experts to determine the most suitable approach.
-
Q2: How do Creative Biolabs' RO assays compare to other target engagement methods?
A2: While other methods may indicate target engagement, our RO assays directly quantify the percentage of receptors bound by your therapeutic agent. This provides a precise, quantitative measure crucial for dose optimization and PK/PD correlation, offering a direct assessment of binding that complements other functional assays.
-
Q3: Can Creative Biolabs' RO assays be adapted for different sample types, such as patient biopsies?
A3: Absolutely. Our RO assay platforms are adaptable to various sample types, including cultured cells, primary cells, and clinical samples like peripheral blood mononuclear cells (PBMCs) or tissue biopsies. Our team has extensive experience in handling diverse biological matrices to ensure accurate and reliable results.
Contact Us
Creative Biolabs is your trusted partner for advanced Receptor Occupancy Assay services. Our commitment to scientific excellence and client success ensures that you receive the highest quality data to drive your drug development forward. Please contact us for more information and to discuss your project.
References
- Aru, Basak, and Gulderen Yanikkaya Demirel. "Flow Cytometry: A Versatile and Powerful Tool for Drug Discovery and Development". Pharmedicine Journal, vol. 1, no. 1, Feb. 2024, pp. 1-19, doi:10.62482/pmj.5.
- Chang, Xiao L et al. "CCR5 Receptor Occupancy Analysis Reveals Increased Peripheral Blood CCR5+CD4+ T Cells Following Treatment With the Anti-CCR5 Antibody Leronlimab." Frontiers in Immunology vol. 12 794638. 19 Nov. 2021, doi:10.3389/fimmu.2021.794638.
- Distributed under Open Access License CC BY 4.0, without modification.
- Distributed under Open Access License CC BY 4.0. The original image was modified by extracting and using part D, and the title was changed to "Assay for CCR5 receptor occupancy".
For Research Use Only.
