Single Dose Study
Creative Biolabs performs all aspects of preclinical pharmacokinetic (PK) and toxicokinetics (TK) evaluations in accordance with Good Laboratory Practice (GLP) procedures. Our integrated in vivo PK/TK platform can seamlessly perform in vivo sample generation as well as bioanalysis. To predict human drug dose and evaluate drug toxicity, Creative Biolabs provides single dose studies in various animal species.
Toxicokinetics (TK) study is the generation of kinetic data for systemic exposure and toxicity assessment of drugs. Creative Biolabs provides single dose study for different dose levels in animal models. The single dose study is useful in selection of dose, the evaluation of toxicological mechanism, as well as for setting safety dose level in clinical trials.
Overview of Single Dose Studies
We can perform single dose study in both rodent and non-rodent models. Plasma, whole blood, urine, and other materials can be taken for the analysis. Results from single dose study may help in the choice of the formulation as well as in the prediction of rate and duration of exposure. If necessary, we provide additional toxicokinetic studies by using low dose level, intermediate dose level, and high dose level to answer specific questions raised by the initial dose study. Here, toxicokinetic evaluation takes place at various time-points for each new dose level. Such an evaluation is especially useful if higher-dose emesis occurs.
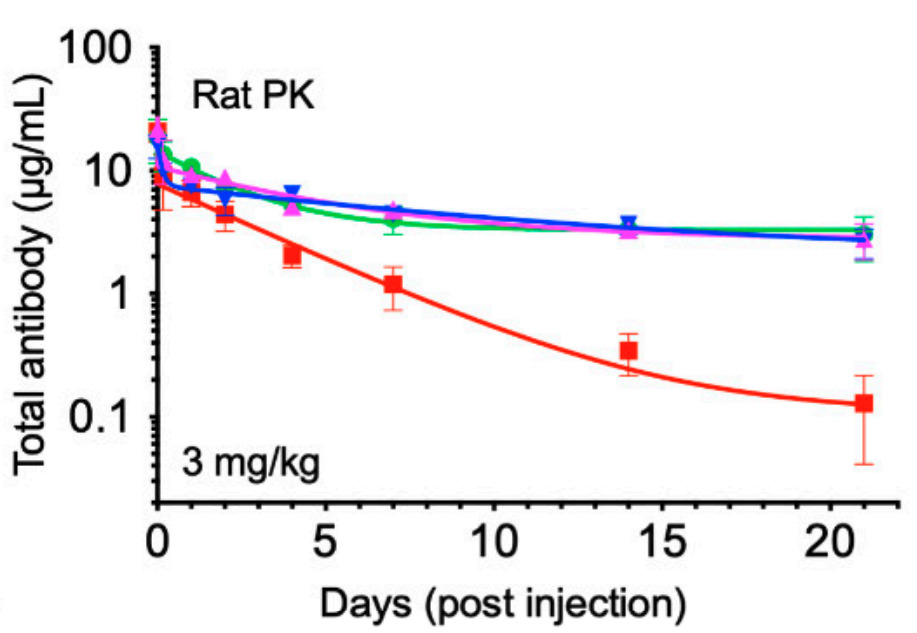 Fig. 1 Pharmacokinetic study in SD rats after a single intravenous ADC dose. 1
Fig. 1 Pharmacokinetic study in SD rats after a single intravenous ADC dose. 1
Design of Individual Studies
Creative Biolabs designs individual studies tailored to your need. Our toxicity studies subject to GLP and take several principles into consideration for the sake of safety and accuracy.
- Quantification of exposure: The exposure might be represented by plasma (or serum or whole blood) concentrations or the AUCs of the parent compound and/or metabolite(s).
- Justification of time points for collecting body fluids in concomitant TK studies.
- Dose level setting: Low dose levels, intermediate dose levels or high dose levels.
- Determination of the number of tested animals: Performed either in all or a representative proportion, both male and female animals.
- Determination of route of administration.
- Metabolites determination.
- Statistical evaluation of data.
Analytical Methods
We use various analytical methods to evaluate plasma (or whole blood or serum) concentrations of pharmaceuticals with adequate sensitivity and precision. Our validated analytical methods conform to Good Laboratory Practice (GLP). Methods used in such studies include:
- Gas chromatography
- HPLC
- LC
- LC-MS
- LC-MS-MS
- Capillary electrophoresis
Available Species
- Mice
- Rats
- Rabbits
- Guinea pigs
- Dogs
- Mini pigs
- Non-human primates
Creative Biolabs evaluates and reports all the data within a project in the same consistent way. We have internal knowledge on the ins and outs of the pharmacokinetic characteristics.
For more detailed information, please feel free to contact us or directly sent us an inquiry.
Reference
- Conilh, Louise et al. "Exatecan Antibody Drug Conjugates Based on a Hydrophilic Polysarcosine Drug-Linker Platform." Pharmaceuticals (Basel, Switzerland) vol. 14,3 247. 9 Mar. 2021, doi:10.3390/ph14030247. Distributed under Open Access license CC BY 4.0. The image was modified by extracting and using the A part of the original image.
For Research Use Only.
