Using Enzyme Inhibitor to Intervene Host Biosynthesis Pathway
The application of chemical tools to inhibit glycosylation offers a powerful approach to study glycan functions and performs as a starting point for drug discovery. There’re various types of inhibitors studied, including natural products, small molecules, substrate-based tight-binding inhibitors, glycoside primers, and many others. As a reliable expert in antibody markets, Creative Biolabs has collected numerous inhibitors through screening chemical libraries and is capable of rationally designing inhibitors based on three-dimensional structures of enzymes.
Glycosylation & Enzyme Inhibitors
Antibody glycosylation is the result of multiple stepwise actions. Many inhibitors are able to modulate the glycosylation of monoclonal antibodies (mAbs) have been reported. Enzyme inhibitors arresting mAbs in the intermediate glycoforms can prevent the additions of outer arm sugar residues. For example, the endoplasmic reticulum (ER) α-mannosidase inhibitors, deoxymannojirimycin and kifunensine produce the high mannose glycoform (e.g.Man9GlcNAc2), while Golgi α-mannosidase II inhibitors swainsonine produces hybrid glycoforms (e.g. GlcNAcMan5GlcNAc2Fuc).
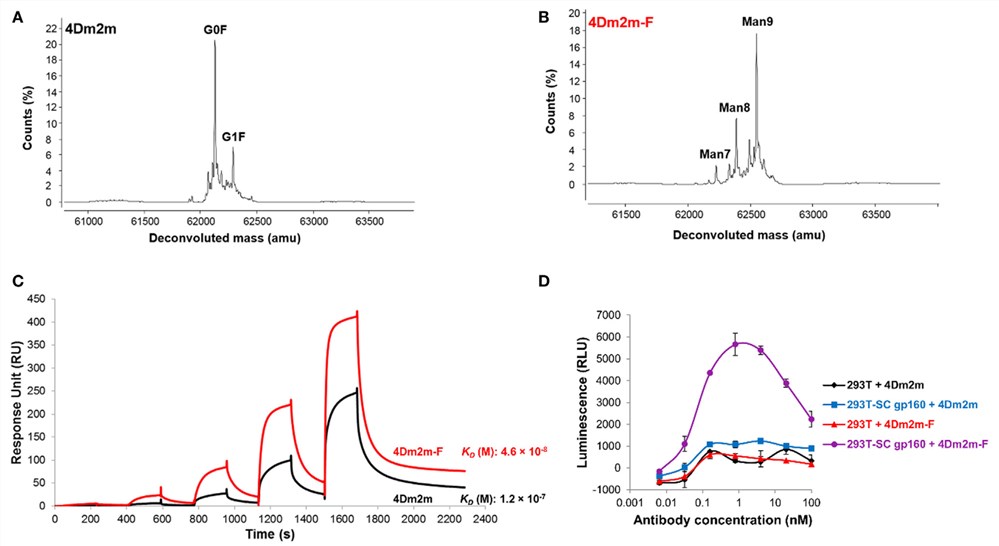 Fig.1 The endoplasmic reticulum α-mannosidase inhibitors (kifunensine) are used to produce the high mannose glycoform with low fucose.1
Fig.1 The endoplasmic reticulum α-mannosidase inhibitors (kifunensine) are used to produce the high mannose glycoform with low fucose.1
- N-glycosylation
Treatment of cells with inhibitors of enzymes that synthesize N-linked glycans leads to the production of glycoproteins embracing missing or altered chains. This method is useful for determining the potential functional role of this glycan on specific proteins or intact cells.
The use of inhibitors to prevent N-glycosylation of proteins in cultured cells contains two steps. First, the optimal concentration of inhibitor for the experiment is examined by monitoring [(35)S]methionine incorporation as a measure of protein biosynthesis. Then, the ability of inhibitors to hinder oligosaccharide processing is analyzed by testing cells labeled with [(3)H]mannose using endo H digestion or trichloroacetic acid (TCA) precipitation.
- O-glycosylation
The O-glycosylation of proteins participates in many biological processes. Removing the functions of O-linked glycans requires glycosyltransferase (Gtf) inhibitors suitable for use in cells.
Here, a general protease-protection assay is described, and in which a peptide labeled with a fluorescence resonance energy transfer (FRET) pair is subjected to glycosylation and later treated with a protease that distinguishes glycosylated and non-glycosylated peptides. This approach is implied to be adapted to monitor the activity of O-GlcNAc transferase (OGT), a Gtf involved in intracellular signaling, and two O-GalNAc transferases (ppGalNAc T1 and T2). It may be possible to use this assay to discover inhibitors for different Gtfs that catalyze O-glycosylation.
Enzyme Inhibitor Design & Custom Services
- Neuraminidase (Sialidase) Inhibitors
Investigations of influenza neuraminidase testify the power of rationally designed drugs, and Fig.2 exhibits the structures of influenza neuraminidase inhibitors.
Visual inspection of influenza neuraminidase with the inhibitor bound indicates that two glutamate (Glu) residues lined a pocket near Carbon 4 (C4) of the sialic acid analog. The pocket was open, suggesting that a substituent at this position might be accepted, at least sterically. A substrate analog was produced has remarkable influenza neuraminidase inhibitor potency (Ki value of 10-11 M). The analog is nearly a million folds less potent on human sialidases, resulting in its function as an anti-influenza drug. However, it does not work on bacterial sialidases because of the abundant arginine (Arg) groups at equivalent pockets.
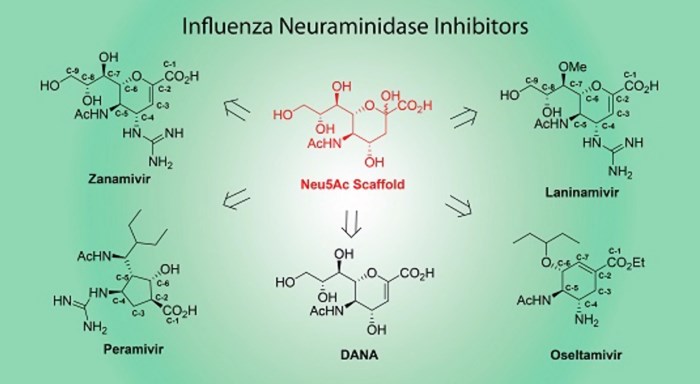 Fig.2 The structures of influenza neuraminidase inhibitors.2
Fig.2 The structures of influenza neuraminidase inhibitors.2
- Sulfotransferase Inhibitors
A large number of Golgi sulfotransferases installs sulfate esters on various glycans using phosphoadenosine-5’-phosphosulfate (PAPS) as an active sulfate donor. Today, high-resolution crystal structures are available for both glycan-modifying sulfotransferases and soluble drug detoxifying sulfotransferases. These enzymes have a conserved fold in common that can bind PAPS. A set of small aromatic compounds, and some disaccharide analogs of GlcNAc-6-sulfotransferase substrates have inhibitory activities. And screening targeted libraries of purine derivatives yielded compounds with high selectivity towards individual sulfotransferases, implying that subtle differences in the PAPS-binding sites can be studied.
With the understanding of glycobiology, Creative Biolabs can exploit easy-to-access techniques to design novel enzyme inhibitors for specified N-linked glycans or O-linked glycans, based on the crystal structure of the corresponding enzyme. Besides, we already possess a huge small molecule library for inhibitor screening. If you’re interested in our platforms, please contact us for more information.
References
-
Li, Wei, et al. "Crystallizable fragment glycoengineering for therapeutic antibodies development." Frontiers in immunology 8 (2017): 1554.
Distributed under Open Access License CC BY 4.0, without modification. -
Laborda, Pedro, Su-Yan Wang, and Josef Voglmeir. "Influenza neuraminidase inhibitors: synthetic approaches, derivatives and biological activity." Molecules 21.11 (2016): 1513.
Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only.
