Virus Neutralization Assay
Creative Biolabs has brought a full range of in vitro cell-based assays in an effort to help our clients better understand the characteristics and mechanism of action of their therapeutic antibody candidates. Particularly, we now offer professional service of virus neutralization assays for studying the potency or blocking the activity of anti-virus antibodies.
Antiviral Monoclonal Antibodies
Monoclonal antibodies (mAbs) are increasingly being considered as agents to fight severe viral diseases. They are selected and used based on their virus-neutralizing activity and/or cell-killing activity to blunt viral propagation via direct mechanisms. So far, most antiviral mAbs with therapeutic potential have initially been selected for their ability to neutralize virions via the recognition of viral surface antigens essential for receptor binding and/or entry into host cells. However, besides direct targeting of viruses, these antibodies can also trigger effector functions mediated by their Fc fragment, eliciting complement-dependent cytotoxicity (CDC), antibody dependent cellular phagocytosis (ADCP), and antibody-dependent cell-mediated cytotoxicity (ADCC) to eliminate infected cells displaying viral antigens at their surface. These characteristics have demonstrated that antiviral antibodies represent promising, high-added-value therapeutic agents.
Virus Neutralization Assay
Due to the importance of virus neutralizing activity for antiviral antibodies, it is necessary to evaluate the potential of antibody candidates to neutralize the target viruses. Virus neutralization assay is an in vitro test to detect the potency of antibodies to block virus infectivity. This assay has been widely used to target a number of viruses, such as H5N1 influenza virus, human immunodeficiency virus (HIV), herpes simplex virus (HSV), cytomegalovirus (CMV), hepatitis C virus (HCV), Ebola virus, and so on.
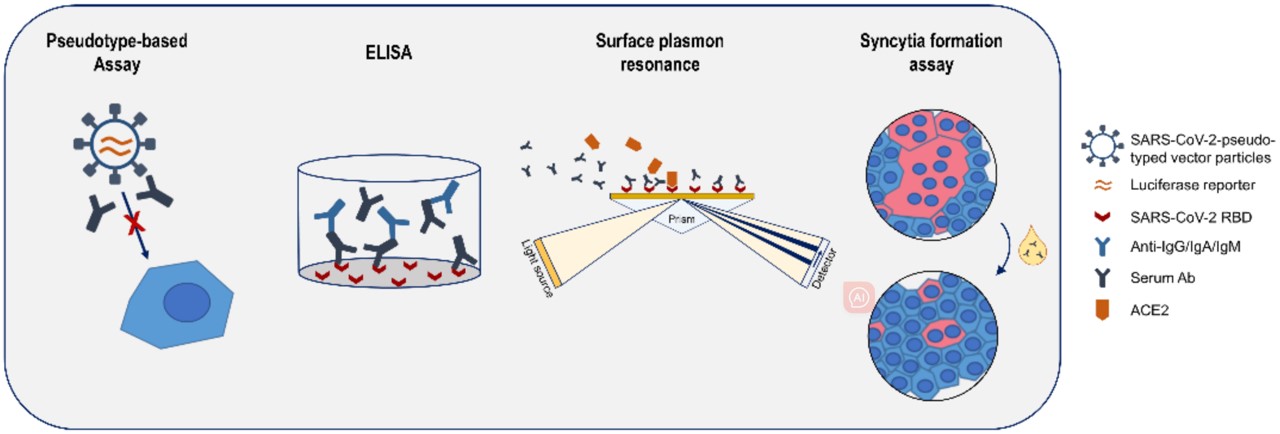 Fig. 1 Pseudotype-based viral neutralization assay.1
Fig. 1 Pseudotype-based viral neutralization assay.1
Virus Neutralization Assay Provided by Creative Biolabs
The virus neutralization assay is performed through co-incubation of the virus, antiviral antibodies, and target cells. Infectivity is identified by the presence of a cytopathic effect on target cells, or alternatively reduction of proliferative effects. The plaque reduction neutralization test (PRNT) is a gold standard method to measure the neutralization potency of antiviral antibodies. For test materials, we can deal with both purified antibodies and rough samples. Moreover, we offer a full menu of pathogenic viruses and pseudotyped viruses that are available to help you find the most suitable target. Appropriate controls are applied to ensure assay validity.
Detection Approaches for Virus Neutralization Measurement
| Plaque Reduction Neutralization Test (PRNT) | Manually quantifying the number of viral plaques formed in cell monolayers through plaque reduction neutralization testing (PRNT) using crystal violet staining in 6- or 12-well plates. |
|---|---|
| Focus-Reduction Neutralization Test (FRNT) | Utilizing immunostaining after viral infection to visualize infected cells and identify specific viral proteins in "foci" using automated imaging systems. |
| Digital Automated Microscopy | Automatically capture fluorescent images of cells treated with potential neutralizing antibodies targeting specific epitopes, and quantify the binding signals through fluorescence analysis software. |
| Enzyme-Linked Immunosorbent Assay (ELISA) | Evaluating fluorescent signals to determine the efficacy of neutralizing antibodies on binding to specific epitopes involved in neutralization. |
| Flow Cytometry | Automatically analyze and quantify the number of infected cells using fluorescent probes. |
| Microneutralization Assays | Utilizing a cutting-edge plate imager, we are able to quickly capture comprehensive data on virus neutralization by efficiently imaging the entire microplate in both bright field and fluorescence, allowing for detailed analysis of counts, morphology, and intensity measurements. |
Representative Data
This study outlines the creation, fine-tuning, and assessment of a live virus microneutralization method tailored to target severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The experimental procedure involves incubating SARS-CoV-2 clinical isolates with sequentially diluted antibodies before introducing the mixture to Vero E6 cells.
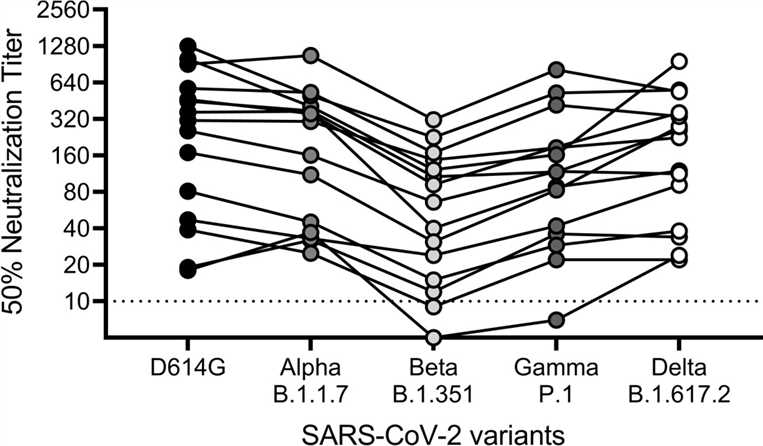 Fig.2 Concerning SARS-CoV-2 variants' virus neutralization.2
Fig.2 Concerning SARS-CoV-2 variants' virus neutralization.2
Features
- Comprehensive data report
- Fast turnaround time
- Amenable to customized services
- Competitive prices
Our exhaustive service of antibody functional assays can guarantee our clients with the most compelling and reproducible outcomes, offering absolute confidence for subsequent investigations. Additionally, Creative Biolabs has built a team of experienced scientists with facilities and processes designed specifically to provide the best strategy and protocols customized to antibody functional characterization services. For more detailed information, please feel free to contact us or directly sent us an inquiry.
Frequently Asked Question
Q1: How to accomplish serum virus neutralization assay?
A1: The serum virus neutralization (SVN) assay is a laboratory test used to determine the presence of functional antibodies in the bloodstream that can prevent a virus from infecting cells. This assay involves exposing the virus to a sample of serum containing antibodies, and then measuring the ability of the antibodies to neutralize or inhibit viral infection. By analyzing the extent of virus neutralization, we can quantify the level of protection provided by the antibodies. Cutting-edge techniques such as digital automated imaging systems have revolutionized the SVN assay, allowing for faster and more accurate analysis of antibody response.
References
-
Herrlein, Marie-Luise, et al. "Comparative Investigation of Methods for Analysis of SARS-CoV-2-Spike-Specific Antisera." Viruses 14.2 (2022): 410.
Distributed under Open Access License CC BY 4.0. The original image was modified by extracting and using a part, and the title was changed to “Pseudotype-based viral neutralization assay”. -
Frische, Anders, et al. "Optimization and evaluation of a live virus SARS-CoV-2 neutralization assay." PLoS One 17.7 (2022): e0272298.
Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only.
