AAV Vector Design for Adenosine Deaminase Deficiency
As a worldwide leader in gene therapy, Creative Biolabs offers high-quality customized service covering the entire gene therapy development process to best suit your technical, program, and budget requirements. Our customer-oriented services include gene delivery vectors' design, optimization & construction, potency tests, safety and toxicology analysis, solutions of specific gene therapy development for diseases, etc. Adeno-associated virus (AAV) has become a powerful vehicle because it elicits minimal immune responses and mediates long-term transgene expression in a variety of cell types.
Adenosine Deaminase Deficiency
Adenosine deaminase (ADA) deficiency is best known as severe combined immunodeficiency (SCID), caused by mutations in the gene encoding ADA. Patients display typical clinical and immunological manifestations of SCID. Currently, therapies are available to target these immune disorders, with varying degrees of clinical improvement in treated patients. However, increasing evidence indicates that ADA deficiency can significantly impact non-immune organ systems. In recent years, gene therapy has made significant progress in treating ADA deficiency and its most common manifestation, severe combined immunodeficiency.
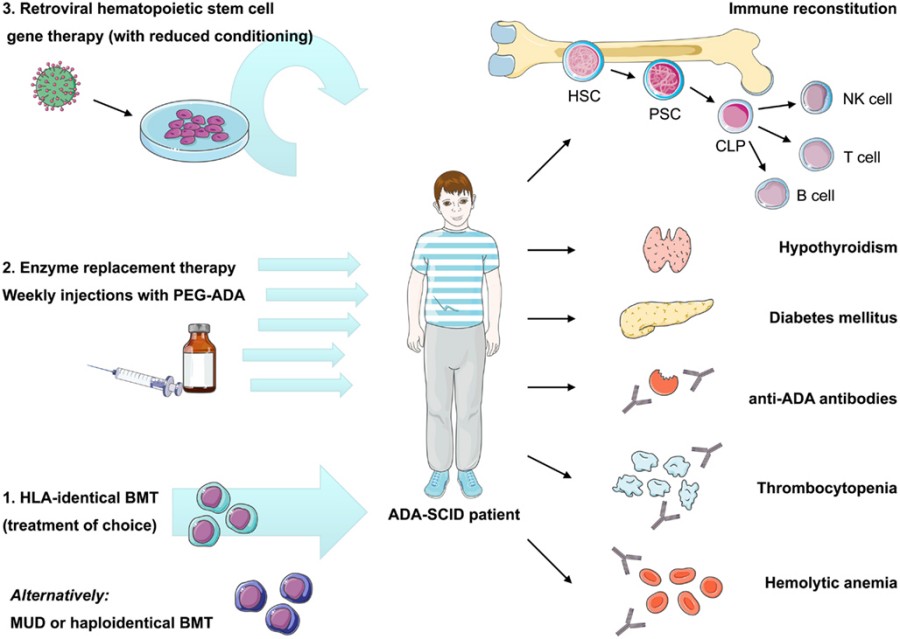 Figure 1 Current therapeutic options in ADA-SCID and reported autoimmune manifestations after treatment.1
Figure 1 Current therapeutic options in ADA-SCID and reported autoimmune manifestations after treatment.1
Gene Therapy for Adenosine Deaminase Deficiency
Over nearly two decades, gene therapies for ADA-SCID have exclusively involved retroviral vectors targeted to lymphocytes and hematopoietic progenitors. However, the risk of insertional mutagenesis and subsequent leukemogenesis cast numerous doubt upon the safety and efficiency of retroviral vectors. An alternative gene therapy for ADA-SCID may utilize recombinant AAV vectors in vivo, with numerous target tissues and relatively low immunogenicity, to foster ectopic expression and secretion of adenosine deaminase. There are lots of characteristics of AAV vectors guarantee their safety and effectiveness, including but not limited to:
- AAV vectors are nonpathogenic with relatively low immunogenicity compared with other viral vectors;
- AAV vectors possess broad tissue tropism and ability to transduce both dividing and nondividing cells;
- AAV vectors are capable of long-term persistence within host cells, facilitating long-term transgene expression and reducing the risk of insertional mutagenesis;
- AAV vectors show a high degree of transduction efficiency into different cell types in vitro, but also efficacy and safety in various small and large animal models in vivo.
AAV Vector Sequence Design
-
Phase I
Capsid Selection and Engineering
Choosing the appropriate AAV serotype is a key factor in the success of ADA-SCID treatment. Different natural serotypes exhibit different tissue tendencies and transduction efficiencies, among which AAV2, AAV6, AAV8, and AAV9 are particularly associated with hematopoietic and immune targets.
-
Phase II
Promoter Optimization and Expression Cassette Design
The selection of promoters is crucial for achieving stable, long-term, and appropriate expression of ADA enzymes. Strong constitutive promoters, such as CMV (Cytomegalovirus), can drive high-level expression of ADA.
-
Phase III
Transgene Engineering and Codon Optimization
ADA transgenes themselves have undergone extensive optimization to maximize their therapeutic potential. This includes replacing less commonly used codons with more commonly used ones, thereby significantly improving translation efficiency. This strategic approach ensures that even low-level transgenic expression can produce physiologically relevant amounts of functional ADA enzymes, thereby providing long-lasting therapeutic effects.
Comparison of Vector Systems for ADA-SCID Gene Therapy
| Vector Type | Advantages | Limitations |
|---|---|---|
| Gamma-retroviral | Permanent integration, stable expression | Insertional mutagenesis risk, complex production |
| Lentiviral | Broender tropism, safer integration profile | Higher production costs, complex regulation |
| AAV | Low immunogenicity, high safety profile | Limited packaging capacity, pre-existing immunity |
Core Services at Creative Biolabs
Based on our advanced technology and abundant experience, Creative Biolabs provides flexible solutions about AAV-based gene therapy through our offer to select stand-alone services or executing projects in an integrated manner. Our gene delivery vectors library wholly covers all gene delivery vectors, including viral (lentiviruses, adenoviruses, adeno associated viruses) and no-viral vehicles (polyplexes, stem cells, dendrimers, bacteria, dendritic cells, etc.). All of these vectors can transfer precise amounts of foreign gene products to each target cell, sequentially allowing expression without causing cytotoxicity. In addition, our gene delivery platform enables AAV design, AAV purification, AAV titration, AAV toxicity and safety determination to meet every specific demand.
Our Service Process
The development of therapeutic AAV vectors is a systematic process. Our workflow is designed to ensure transparency and collaboration, ensuring client involvement at every step of the R&D process.
-
Discovery and Conceptualization
We begin with a deep understanding of our client's project goals, target cell type (in this case, hematopoietic stem cells), and therapeutic needs. Our experts design the vector architecture, including the selection of an AAV serotype (e.g., AAV6 or its novel variants), a promoter driving ADA expression, and a codon-optimized transgene cassette. -
Molecular Engineering and Vector Production
- Gene Synthesis: The optimized ADA gene is synthesized and cloned into a proprietary AAV shuttle plasmid with necessary ITRs.
- Transient Transfection and Production: Recombinant AAV vectors are produced in HEK293 cells using a reliable three-plasmid transient transfection system, suitable for scalable research and preclinical use.
- Downstream Processing: The viral lysate is purified through a multi-step process involving clarification, nuclease treatment to eliminate plasmid DNA, and chromatography to isolate AAV particles.
-
Quality Control and Characterization
- Titer Determination
- Purity Assessment
- Sterility and Endotoxin Testing
Reasons to Choose Creative Biolabs
Scientific Expertise
Our team of PhD scientists has extensive experience in virology, molecular biology, and gene therapy. This deep knowledge ensures that every vector is designed with scientific excellence as the top priority.
State-of-the-Art Technology
We utilize the latest technologies for gene synthesis, vector production, and quality control, ensuring the highest standards of quality and purity.
Customized Solutions
We understand that every project is unique. Our services are highly customizable, and we work collaboratively with our clients to meet their specific requirements.
Proven Track Record
Our vectors have been used in numerous successful preclinical studies, and we have a long history of satisfied clients who have achieved their research milestones with our support.
Frequently Asked Questions
Q: Why is AAV vector suitable for ADA deficiency gene therapy?
A: AAV vectors have many advantages in ADA-SCID applications, including high safety, low pathogenicity and immunogenicity; Can efficiently transduce dividing and non-dividing cells related to ADA-SCID; Long term transgenic expression can be maintained for many years after a single administration. The nonintegrated nature of AAV vectors reduces the risk of genotoxicity while still providing sustained enzymatic correction.
Q: How to solve the problem of limited packaging capacity of AAV vectors?
A: Adopting multiple strategies to overcome the packaging capacity limitation of AAV vectors of approximately 4.7 kb: (1) using synthetic promoters with reduced size but unchanged activity for minimalist promoter design; (2) The self-complementary genome synthesized by bypassing the second strand can initiate expression more quickly; (3) Overlap mediated recombination systems for larger genes; And (4) codon optimization, reducing transgenic size without changing the protein sequence. For ADA genes, due to their appropriate packaging size, we can add additional regulatory elements to enhance their expression.
Q: What strategies do you use to alleviate immune responses against AAV vectors?
A: Our comprehensive immunogenic relief methods include: (1) evading pre-existing immunity through capsid engineering modifications; (2) Remove empty capsids during the purification process to reduce antigen load; (3) Using tissue-specific promoters to minimize off target expression to the greatest extent possible; (4) To achieve transgenic de immunization by removing immune dominant epitopes; And (5) using proprietary dosing regimens to regulate immune responses. For patients with pre-existing high immunity, we can develop novel capsid variants that can evade neutralization reactions.
Q: What is the difference between scAAV and ssAAV in treating ADA deficiency?
A: ScAAV vectors contain double stranded DNA, so they do not require host cell DNA synthesis to initiate transcription. This makes them an ideal choice for quiescent HSPCs (with low DNA replication rates), mediating ADA expression 2-3 times higher than ssAAV. However, the packaging capacity of scAAV is relatively small (about 2.4 kb, while ssAAV is 4.7 kb), making it the most suitable for compact transgenic boxes (such as codon optimized ADA without large enhancers).
Q: What is the typical turnaround time for a customized AAV vector design project?
A: The schedule varies due to the complexity of design and production scale. However, we usually provide a detailed project schedule during the initial consultation period to ensure transparency and timely delivery.
Customer Review
"When our lab embarked on a challenging gene therapy project for ADA-SCID, we knew we needed a partner with unparalleled expertise in AAV vector engineering. The team at Creative Biolabs exceeded every expectation. Their in-depth understanding of hematopoietic stem cell biology and AAV serotype tropism was evident from our very first discussion. They didn't just follow our instructions; they provided critical, data-driven insights that led to the development of a superior vector."
— Dr. Julian Hayes, Director of R&D
Connect with Us Anytime!
Creative Biolabs provides high-quality AAV-based services for basic research and clinical applications of ADA deficiency. Novel molecular strategies and experienced engineering techniques are applied to offer satisfactory products or services to promote your brilliant success. If you have any requirement, please feel free to contact us for more information.
Reference
- Sauer A V, Brigida I, Carriglio N, et al. Autoimmune dysregulation and purine metabolism in adenosine deaminase deficiency. Frontiers in immunology, 2012, 3: 265. https://doi.org/10.3389/fimmu.2012.00265 (Distributed under Open Access license CC BY 4.0, without modification.)
