AAV Vector Design Service for Alpha-1 Antitrypsin Deficiency
Alpha-1 antitrypsin deficiency (A1ATD) remains the most common inherited metabolic disorder of the lung and liver. Conventional protein-augmentation therapy demands weekly IV infusions, the expenditure is extremely large, and cannot halt progression to emphysema or liver fibrosis. High-fidelity, liver-tropic rAAV particles are engineered to deliver a codon-optimized SERPINA1 expression cassette under transcriptional control of a hepatocyte-selective promoter, ensuring robust yet tissue-restricted synthesis of functional α-1-antitrypsin. The construct incorporates a split-polyadenylation signal and cPPT/CTS hyper-element to accelerate nuclear import and transgene expression, while miR-122 target sites are embedded to suppress off-target transcription in non-parenchymal cells. An AAV3B-derived capsid variant—subjected to surface-loop remodelling and glycan shield insertion—exhibits reduced neutralizing antibody binding without compromising heparan-sulfate attachment. Integrase-null Rep/Cap plasmids and self-inactivating ITRs abolish integration competence, and high-stringency, helper-free production yields vectors with undetectable replication-competent AAV and minimal residual host DNA. The resulting episomal genomes persist quiescently within hepatocyte nuclei, driving sustained antiprotease secretion that protects pulmonary elastin from neutrophil elastase–mediated degradation, yet decline gradually upon hepatocyte turnover—providing a transient, non-mutagenic therapeutic window aligned with current safety expectations for in vivo gene therapy.
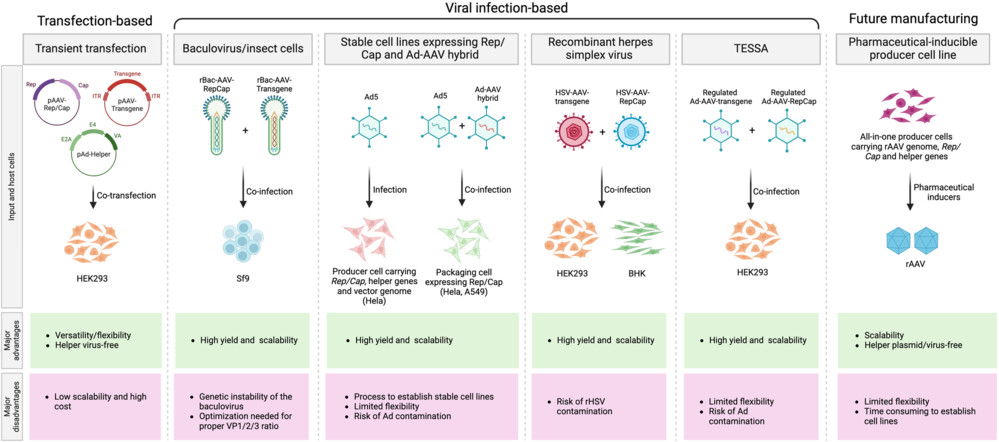 Fig.1 Current approaches to manufacture rAAV1,2
Fig.1 Current approaches to manufacture rAAV1,2
The governing axiom we uphold is that durable correction of protease–antiprotease imbalance need not etch a permanent signature into the host genome. Creative Biolabs's AAV-A1AT service unites deep hepatocyte tropism knowledge, cryo-EM-guided capsid resurfacing, and single-cell secretion bar-coding into a seamless pipeline that dissolves every barrier between your pulmonary protection hypothesis and a sequence-verified, liver-restricted AAV vector. Using serum-free, helper-free packaging lines optimized for high-capacity AAV3B capsid expression, we deploy combinatorial cPPT/CTS libraries, miR-122 shield matrices, and charge-inversion surface surgery to craft particles with supranuclear titers, undetectable RCL background, and heparan-sulfate engagement kinetics that trigger SERPINA1 expression only upon hepatocyte entry. These integration-null vectors, delivered endotoxin- and aggregate-free, illuminate target-positive hepatocytes in patient-derived organoids and orthotopic xenografts with cinematic brightness, enabling mechanistic studies to sprint from bench to publication-ready datasets without genomic footprint or procedural delay.
Your Strategic Advantage in Gene Therapy
Guiding the complexities of liver-tropic AAV design shouldn't delay your correction of Alpha-1 Antitrypsin Deficiency. We deliver the expertise, capsid technology, and genuine partnership to help you realize your research plan.
Collaborative Scientific Partnership
Our PhD-level team acts as an extension of your group, providing proactive consultation on codon optimization, promoter strength, and enhancer placement.
Seamless Scalability
Our platform evolves alongside your program, maintaining unwavering vector quality and performance whether you're testing a new idea in a single plate or advancing toward pre-clinical milestone.
Uncompromising Quality & Safety
By embedding safety into every design choice and backing it with exhaustive quality control, we supply vectors ready to satisfy the toughest pre-clinical and clinical standards.
Timeline-Driven Project Management
Recognizing that momentum matters, our project leaders maintain open, real-time dialogue and reliable schedules so your timeline never loses stride.
Our Vector Service Workflow
Our step-by-step, open-framework workflow locks in reliability and progress you can track, turning each stage into a visible checkpoint and a forecast you can trust.
| Phase | Deliverables | How Obtained |
|---|---|---|
| Mutation & Promoter Design | Vector map, codon-optimized hA1AT, regulatory strategy | Use bioinformatics software to design and map the vector structure. HA1AT is Synthesized using gene synthesis services with optimized codon usage for the target organism. Regulatory strategy is Developed based on literature review and experimental data to ensure efficient gene expression. |
| Gene Synthesis & Build | Sanger-verified plasmid, endotoxin-free | Plasmid is synthesized plasmid is sequenced using the Sanger method to confirm accuracy. Plasmid is purified using endotoxin removal columns to ensure it is free from bacterial endotoxins. |
| Small-Scale Pilot | Research-grade AAV, MOI matrix | Research-grade AAV: Produced by transducing cells with the constructed vector and harvesting the AAV particles. Analysis is determined by titering the AAV stock and preparing dilutions for various multiplicities of infection (MOI). |
| Functional QC | ELISA hA1AT secretion, NE-inhibition assay, integration | Measure the secretion of HA1AT protein in cell culture supernatants. NE-inhibition assay is Conducted to assess the inhibitory effect of HA1AT on neuraminidase activity. Analysis through PCR or sequencing to check for vector DNA integration into the host genome. |
| Scale-up & Purification | Different volume; anion-exchange + size-exclusion; endotoxin ≤10 EU/mL | Scaled up production by increasing the volume of the cell culture. Use chromatography techniques to purify AAV particles. Endotoxin ≤10 EU/mL is ensured by further purification and endotoxin removal steps to meet safety standards. |
A Flexible Suite of Services
Creative Biolabs's AAV vector suite integrates state-of-the-art packaging cells, high-density transfection, and proprietary purification techniques to manufacture sequence-verified, replication-incompetent AAV particles. Our service workflow is designed to accelerate your research from concept to in vivo validation seamlessly.
Vector Design & Strategy
Your research objective defines the destination; we architect the AAV vector that navigates the genomic landscape to reach it. Starting with mechanistic modeling of your transgene's expression cassette and continuing through scalable, high-quality production, every design parameter—promoter strength, capsid selection, insulator placement, and safety switch configuration—is optimized against the molecular signature of your target tissue and the clinical timeline of your indication. Regulatory strategy is embedded as a design criterion from day zero, ensuring that each decision—vector backbone, packaging system, and release assay—anticipates CMC expectations and accelerates your path to the clinic.
- Promoter Selection
You choose the expression profile; we engineer the switch. Constitutive powerhouses—CMV for maximal output in HEK293, EF1α for immune cells, CAG for broad tissue coverage. Fine-tuned tissue specificity—Syn1 (neurons), GFAP (astrocytes), Albumin (hepatocytes), or any promoter from our extensive library. Inducible cassettes for temporal control; we optimize the TRE spacing and insulator sequences to eliminate basal leak. In the short term, you receive a promoter activity heat-map in your cell line, plus a ranked recommendation list.
- Multi-cistronic Architectures
Need one vector to do multiple jobs? We handle the stoichiometry for you. 2A peptide linkers (P2A, T2A, E2A)—balanced cleavage efficiency validated by LC-MS. IRES elements—strong (EMCV) for equimolar dual expression or attenuated (CrPV) when downstream gene must lag. "All-in-one" CRISPR arrays—Cas9-2A-BFP-U6-sgRNA-WPRE; we remove cryptic poly-A sites and predict gRNA secondary structure to preserve cutting efficiency. Annotated SnapGene file plus predicted cleavage ratios and experimental validation data from a pilot transduction.
- Safety Modifications
Regulatory reviewers appreciate clean genomes. We handle the details so you don't have to rewrite your IBC protocol. Self-inactivating promoters—deletion of enhancer elements blocks RCL formation; we sequence-verify both 5′ and 3′ junctions. Full biosafety dossier (sequence alignments, RCL assay plan) ready for institutional review.
Cloning & Sequence Verification
- Flexible Input
Have nothing but a FASTA? We'll design promoters, Kozak context, and poly-A signal. Hand-drawn sketch on a napkin? Our bioinformatics team will convert it into an annotated GenBank file in the short term. Existing plasmid that keeps failing? Send the samples; we'll sub-clone the cassette into a high-yield AAV backbone while preserving restriction sites you love. Editable SnapGene file + design rationale report—before we touch a pipette.
- Golden-Gate & Gibson Assembly
Golden-Gate for multi-fragment builds (promoter-ORF-marker in one pot). We optimize overhangs with thermodynamic modeling to give >95% correct colonies. Gibson Assembly for complex inserts >7 kb; we run a proprietary 3-step annealing program that halves the typical error rate. Zero scar junctions mean no unwanted amino acids in fusion proteins—critical for crystallography or antibody labeling. Colony PCR screen data and mini-prep QC gel the same day assembly is complete.
- 100% Sanger Verification
Entire insert, both ITRs, and every junction are covered by overlapping primers. We cross-check against your original design with automated variant calling; you receive a color-coded alignment. If any SNV or indel appears, we re-sequence the batch at no cost—and re-build if the error frequency exceeds 0.05%. Signed sequence certificate ready for patent filing or journal submission.
- Optional Codon-Optimization
Species-specific algorithms for human, mouse, or rat; we balance CAI, mRNA secondary structure, and rare tRNA availability. Cryptic splice-site removal predicted by MaxEntScan; eliminates aberrant transcripts before they start. Immunogenic epitope depletion for in vivo applications—validated by NetMHCpan. Before-and-after expression bar charts in your target cell line, plus a one-page rationale for reviewers.
High-Titer AAV Vector Production for Alpha-1 Antitrypsin Deficiency
Creative Biolabs's comprehensive production platform is designed to streamline the entire process from the moment your sequence-verified plasmid arrives at our facility. By using serum-free suspension growth of HEK293T cells in chemically defined media, it eliminates the variability aspect of the batch with respect to serum, which happens to be an ingredient. High-quality results. Single-use bioreactors allow flexible working volumes that reduce any intervention of cross-contamination and help maintain a timetable for your vector production project as well as keep it prioritized.
- Triple-Plasmid Transfection with Chemical Enhancer
Our gene transfer process employs a triple-transfection protocol, simultaneously delivering the transfer plasmid, packaging construct, and envelope helper. Use a low-toxicity enhancer in the transfection to help stabilize the DNA-lipid complex and facilitate nuclear uptake. Good cytoplasmic trafficking also has to be balanced with cell viability. This enhancer is synthetic; hence, no adventitious agents are introduced. Therefore, it is compatible with research and regulatory workflows.
- Real-Time Metabolite Suite
We keep an eye on important metabolites like glucose, lactate, glutamine, and dissolved oxygen with the help of online sensors. Real-time data goes into a design-of-experiments (DoE) setup that can change feed volume, aeration rate, and transfection stoichiometry on the fly. This setup makes sure to get the most packaging-competent vector genomes while cutting down on non-infectious particles which leads to a higher functional over total capsids ratio all without needing manual tweaking.
- DoE-Driven Optimization
By integrating serum-free suspension culture with real-time DoE control, we achieve AAV supernatants that meet or exceed the functional titers required for modern pre-clinical applications. This method skips the wait times and money spent on old stainless-steel tools. The clear harvest is right away used for further cleaning steps making sure a smooth shift from cell growth to the last vector item letting you keep your mind on your study.
Advanced Purification Pipeline
Our end-to-end purification pipeline starts with the clarified viral harvest and ends with an endotoxin-controlled, ready-to-use vector stock. The process includes three key steps: ion-exchange capture, nuclease polishing, and size-exclusion chromatography. Each step is designed to remove specific impurities while preserving infectious particles.
- Ion-Exchange Capture
Ion-exchange capture leverages the high isoelectric point of the AAV capsid and the negative charge of host-cell DNA. The harvested supernatant is loaded onto a high-flow resin that selectively binds vector particles, allowing contaminating DNA, residual plasmid, and host proteins to flow through. A high-salt wash removes residual DNA, maintaining capsid integrity. This step significantly reduces protein and nucleic acid load, providing a clean feed for subsequent steps.
- Universal Nuclease Digestion
The Universal nuclease is applied under conditions that allow optimal activity of DNase/RNase without lysing viral membranes. It will be removed later by downstream diafiltration so there will not be any foreign proteins in the final product. This will throw out leftover DNA fragments that may cross-react in functional titer assays or those that may elicit an immune response.
- Size-Exclusion Polishing
Size-exclusion chromatography separates intact vector particles from empty capsids and digested oligonucleotides. Using a composite agarose-dextran matrix, particles are fractionated based on hydrodynamic radius. Full vector genomes elute ahead of empty particles, allowing for the collection of a high-quality, genome-containing virion pool. The column is calibrated daily to ensure consistent results.
Comprehensive QC Analytics
Our integrated analytics package assesses each lot at the genomic, protein, functional, and safety levels. Upon completion, you receive a comprehensive certificate summarizing all data and interpretations, ready for institutional review or grant submission.
- Functional Genome Titer
Functional genome titer is determined by digital PCR, probing the vector DNA genome against a single-copy standard. This ensures only encapsidated, competent DNA is scored, providing a direct input for dose–response calculations. Physical particle count is obtained by capsid ELISA, and the particle-to-infectivity ratio is calculated to flag any issues before the product reaches your lab.
- Replication-Competent AAV (RCA) Assay
RCA is detected using vector-specific qPCR targeting the packaging signal and rep region. The assay is performed on both producer supernatant and final bulk, ensuring no recombination events are present. Purity and impurity profiling involve SDS-PAGE, densitometry, and quantitative methods to confirm correct stoichiometry and robust impurity removal.
- Sterility and Endotoxin Testing
Sterility and endotoxin levels are assessed using compendial assays. Endotoxin is measured by kinetic chromogenic Limulus amebocyte lysate, and sterility is confirmed by inoculating anaerobic and aerobic media. Mycoplasma testing ensures no common contaminants are present. All raw data and interpretations are provided, ensuring your downstream experiments are well-supported.
What Our Clients Say
We are dedicated to building long-term partnerships and take pride in delivering exceptional results. See what leading research institutions and biotech companies have to say about our services.
"Faced with the challenge of hepatic specificity in our A1AT project, Creative Biolabs's tailored AAV vectors, driven by a liver-optimized promoter, delivered impressive transduction rates without off-target effects. Their meticulous design process and clear communication turned a complex problem into a streamlined solution."
Dr. Emily Chen
Hepatology Research Team Leader
" Creative Biolabs's expertise in AAV vector design was pivotal for our Alpha-1 Antitrypsin Deficiency study. The vectors showed remarkable tissue specificity and durability. Their service significantly accelerated our research timeline and enhanced our publication quality."
Prof. James Lee
Molecular Therapy Specialist
" The customized AAV vectors from Creative Biolabs exceeded our expectations for treating Alpha-1 Antitrypsin Deficiency. Their focus on safety and efficacy resulted in vectors that were not only effective but also minimized potential liabilities. A true asset to our therapeutic development."
Dr. Lisa Nguyen
Chief Scientific Officer
Frequently Asked Questions
Have questions? Find quick answers here. For more specific inquiries, please don't hesitate to contact us.
Q: What is the primary goal of the AAV Vector Design Service for Alpha-1 Antitrypsin Deficiency?
A: Our primary goal is to create a highly specific and efficient AAV vector that targets liver cells to deliver the SERPINA1 gene, thereby increasing the levels of alpha-1 antitrypsin to treat the deficiency.
Q: What makes your AAV vectors suitable for therapeutic use in Alpha-1 Antitrypsin Deficiency?
A: Our vectors are designed for long-term expression without integration into the host genome, reducing the risk of insertional mutagenesis. They are also produced under conditions that ensure high purity and low immunogenicity, which is crucial for therapeutic applications.
Q: How do you address the issue of pre-existing immunity to AAV vectors?
A: We offer strategies such as using alternative AAV serotypes with lower prevalence of neutralizing antibodies in the population or incorporating immune-evasion sequences into the vector design.
Q: What quality controls are in place for the AAV vectors produced?
A: We perform rigorous quality control assays, including tests for vector purity, concentration, and functionality. This ensures that the vectors are free from contaminants and are capable of effective gene transfer and expression.
Q: Can the AAV vectors be customized for different levels of therapeutic protein expression?
A: Yes, we can adjust the vector design to modulate the level of SERPINA1 expression, allowing for fine-tuning of therapeutic protein levels to meet specific treatment requirements.
References
- Wang, Jiang-Hui, et al. "Adeno-associated virus as a delivery vector for gene therapy of human diseases." Signal Transduction and Targeted Therapy 9.1 (2024): 78. https://doi.org/10.1038/s41392-024-01780-w
- Distributed under Open Access license CC BY 4.0, without modification.
