Custom Adenoviral Vector Production Service
Introduction
Our Custom Adenoviral Vector Production Service accelerates gene therapy development and helps you obtain high-quality, reliable adenoviral vectors through advanced recombinant DNA technology and stringent quality control. Tailored for researchers and biopharmaceutical firms, we deliver optimized vectors to overcome common production challenges, ensuring efficient gene delivery and expression for applications from gene therapy and vaccine development to basic research.
[Discover How We Can Help - Request a Consultation]
Adenoviral Vector Production Service
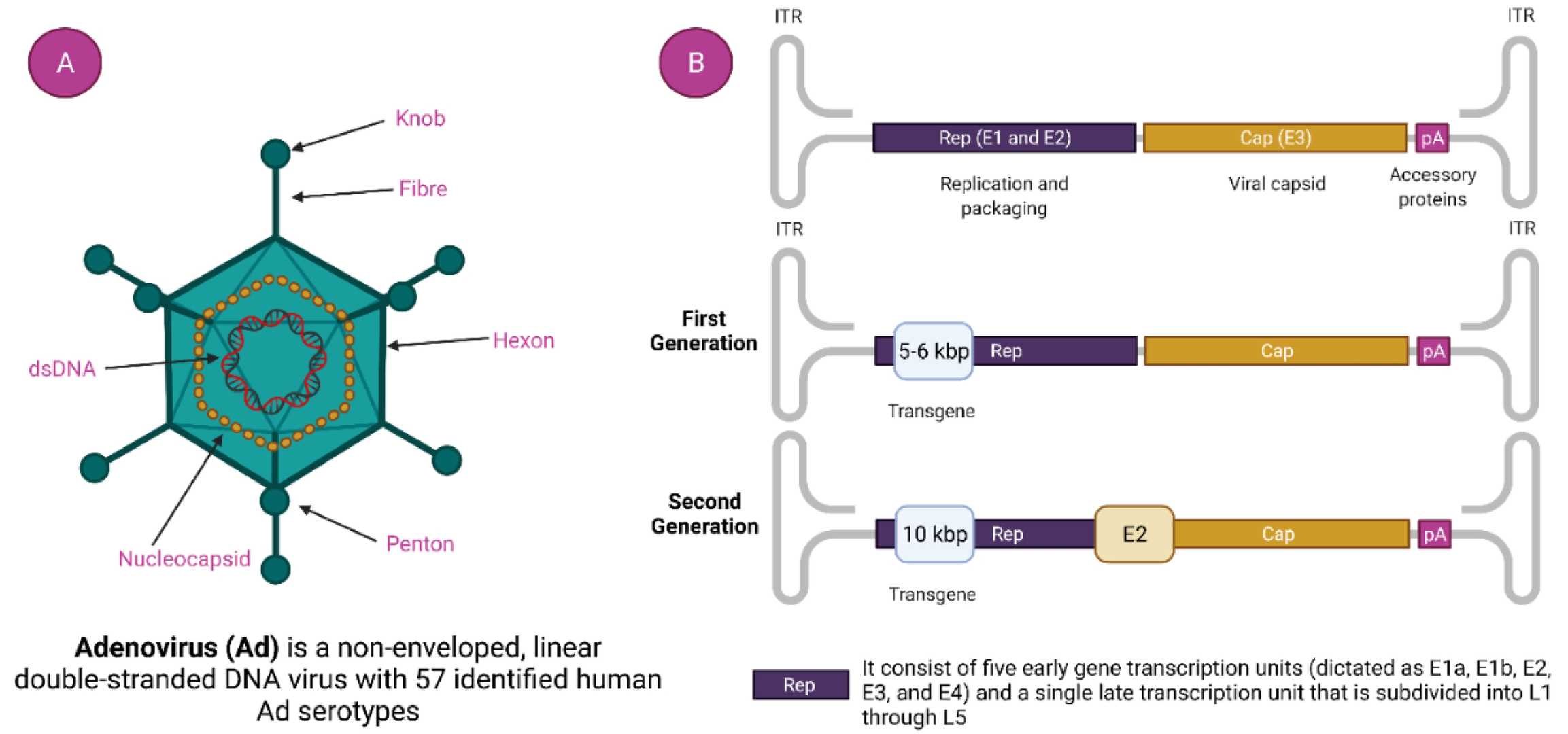 Fig.1 Structure of adenovirus as well as adenovirus vectors.1,3
Fig.1 Structure of adenovirus as well as adenovirus vectors.1,3
Adenoviruses are non-enveloped viruses containing a linear double-stranded DNA genome. Commonly derived from human adenovirus serotypes 2 and 5, these vectors are engineered to be replication-defective for safety in gene delivery. Their structure, characterized by fiber, penton, and hexon-based proteins, facilitates efficient attachment and internalization into a broad range of cell types. This inherent tropism and robust gene expression capacity position adenoviral vectors as critical molecular tools.
Workflow
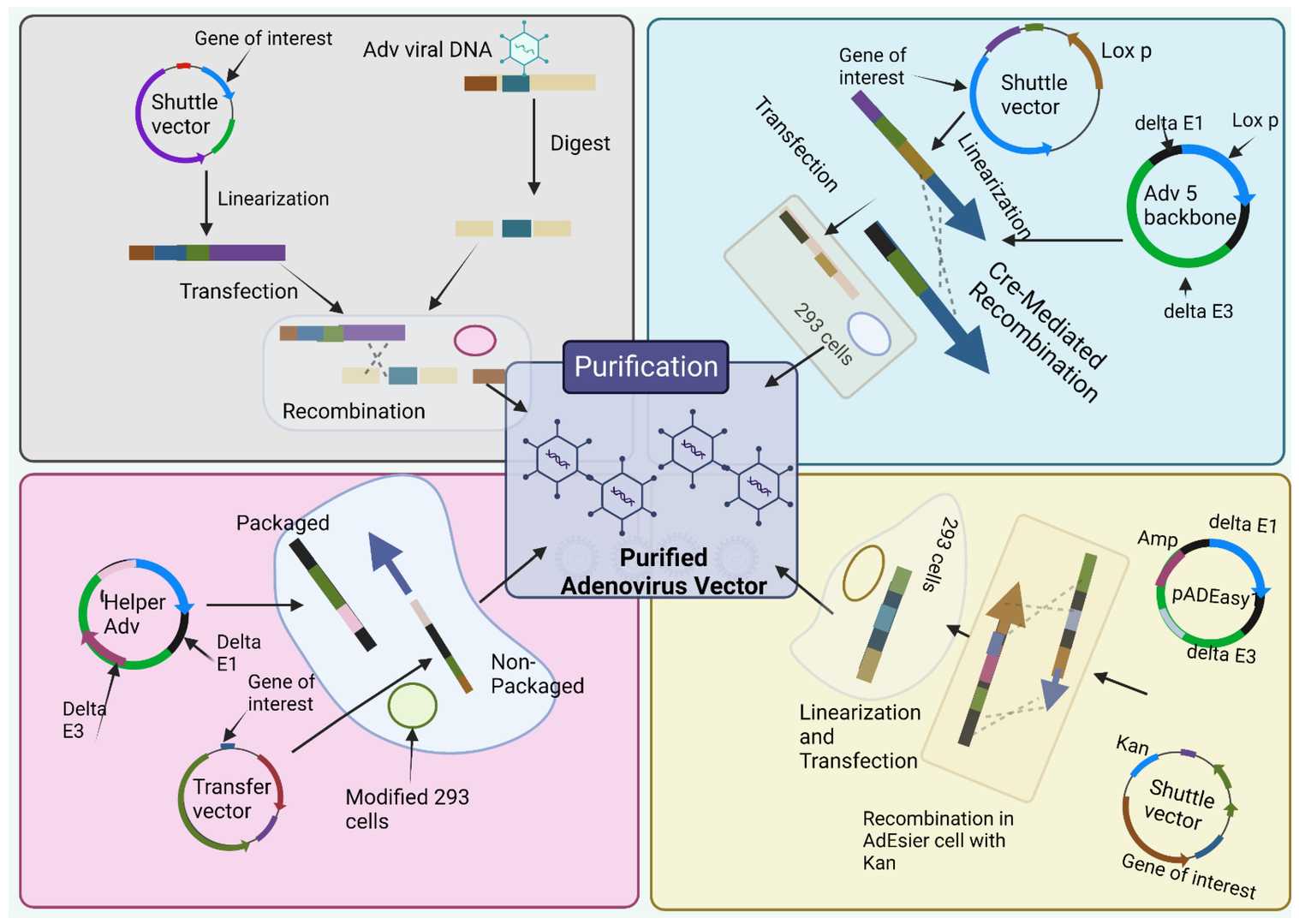 Fig.2 Different adenoviral vector construction methods, including the traditional method, Cre/LoxP-mediated recombination, the AdEasy system, and the use of helper adenovirus for the production of HC.1,3
Fig.2 Different adenoviral vector construction methods, including the traditional method, Cre/LoxP-mediated recombination, the AdEasy system, and the use of helper adenovirus for the production of HC.1,3
-
Produce
Our custom adenoviral vector production uses homologous recombination/direct ligation to clone genes into shuttle vectors, confirmed by sequencing. Transfected into HEK293 cells, the vectors are packaged and amplified via successive infections. Scalable large-scale production in optimized cultures yields high-titer lysates for downstream purification, meeting research to clinical needs.
-
Purification
After production, the crude adenoviral lysate is purified via multi-step processes like cesium chloride ultracentrifugation or anion-exchange chromatography to remove cellular debris, host proteins, nucleic acids, and empty capsids. This yields highly concentrated, pure vectors that minimize contaminant-induced immunogenicity and toxicity, essential for in vitro and in vivo applications.
-
Quality Control
- Titer Determination: Measure infectious particles via plaque assay and genomic copies by qPCR to quantify vector functionality.
- Purity Assessment: Use SDS-PAGE and electron microscopy to eliminate protein contaminants and verify viral integrity.
- Sterility Testing: Rigorously screen for bacterial, fungal, and mycoplasma contamination.
- Endotoxin Testing: Quantify endotoxins in in vivo-grade vectors to ensure safety (levels below regulatory thresholds).
- Functional Validation: Confirm transgene expression and biological activity through in vitro/in vivo assays.
What We Can Offer
Our Custom Adenoviral Vector Production Service delivers high-quality, precisely engineered viral vectors tailored to your research needs, with expert support and customizable solutions.
Why Choose Us?
Unparalleled Expertise
With over 20 years of experience in the field, our team possesses deep scientific knowledge in virology and gene delivery, ensuring optimal vector design and production strategies.
High Transduction Efficiency
Our adenoviral vectors are engineered for high transgene effectiveness, capable of transducing a wide variety of dividing and non-dividing cell types, both in vitro and in vivo.
Large Cargo Capacity
Adenoviruses can carry relatively large foreign DNA fragments, making them ideal for delivering complex genetic constructs.
Robust Immunogenicity for Vaccines
As highlighted in published data, adenoviruses are highly effective in inducing potent immune responses, making them a preferred choice for vaccine development, including candidates for infectious diseases and cancer immunotherapies. They are also more affordable and thermostable than mRNA vaccines.
Stringent Quality Control
Every batch of adenoviral vector undergoes comprehensive quality control, including titer determination, bioburden, mycoplasma, and endotoxin testing, ensuring safety, purity, and consistency.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
Case Study
| Efficiency of Transfection | |
|---|---|
|
|
|
| Apoptosis | Tumor Volume |
|
|
|
FAQs
Q1: How to overcome the problem of immune clearance when adenoviral vectors are used in vivo?
- Serotype modification: rare serotypes (such as Ad35 and Ad48) were used to replace traditional Ad5 to reduce the neutralization of pre-existing antibodies (preclinical data showed that the antibody binding rate decreased by 82%).
- PEGylation: extending the circulating half-life (from <30 min to > 4 h) by covalently binding to the viral surface with polyethylene glycol;
- Immunosuppressive regimen: a short-term low-dose regimen of cyclosporine combined with corticosteroids was provided to suppress the humoral immune response (IgG level decreased by 65%) without affecting the transduction efficiency.
Q2: In the field of gene editing, what are the technical breakthroughs of adenoviral vectors compared with other delivery systems?
A: Our adenovirus vector construction platform offers three major advantages:
- No risk of DNA integration: the use of special gene delivery technology to avoid exogenous DNA insertion mutation.
- Efficient large fragment knock-in: > 5kb gene site-directed insertion;
- Transient expression regulation: rapid degradation after editing within 48 hours, and the off-target rate was 2.7 times lower than that of AAV.
Q3: For solid tumor therapy, how can adenovirus vectors break through the tumor microenvironment barrier?
A: We've developed tumor microenvironment-adapted vectors:
- pH-sensitive capsids that expose penetrating peptides in acidic tumors to enhance cell internalization;
- MMP-responsive capsids with MMP-2 cleavable peptides to release fibrin-targeting ligands for matrix penetration;
- Co-delivery of immunomodulators (cytokines/immune checkpoint inhibitors) to reverse immunosuppressive microenvironments.
Q4: How to balance immunogenicity and safety of adenovirus vectors in vaccine production?
A: Our vaccine optimization strategy:
- Modification of the E3 region: deletion of the E3 region and insertion of the antigen gene to retain the natural adjuvant effect and reduce cytotoxicity;
- Double-epitope display: insertion of HPV L1/L2 epitopes into the H1 loop of capsid fibrin induced 4.3 times higher neutralizing antibody titer than that of the unit vaccine;
- Temperature-sensitive mutants: to construct replication-deficient adenovirus at 40℃ to avoid excessive immune activation under high temperature.
Our Custom Adenoviral Vector Service delivers high-quality, scalable solutions with expert support and strict QC. Contact us today for personalized quotes and accelerate your gene therapy, vaccine, or research projects.
[Click Here to Request a Consultation]
References
- Chavda, Vivek P., et al. "Adenoviral vector-based vaccine platform for COVID-19: Current status." Vaccines 11.2 (2023): 432. DOI: 10.3390/vaccines11020432
- Muhammad, Tahir, et al. "Mesenchymal stem cell-mediated delivery of therapeutic adenoviral vectors to prostate cancer." Stem cell research & therapy 10 (2019): 1-12. DOI:10.1186/s13287-019-1268-z
- Distributed under an Open Access license CC BY 4.0, without modification.

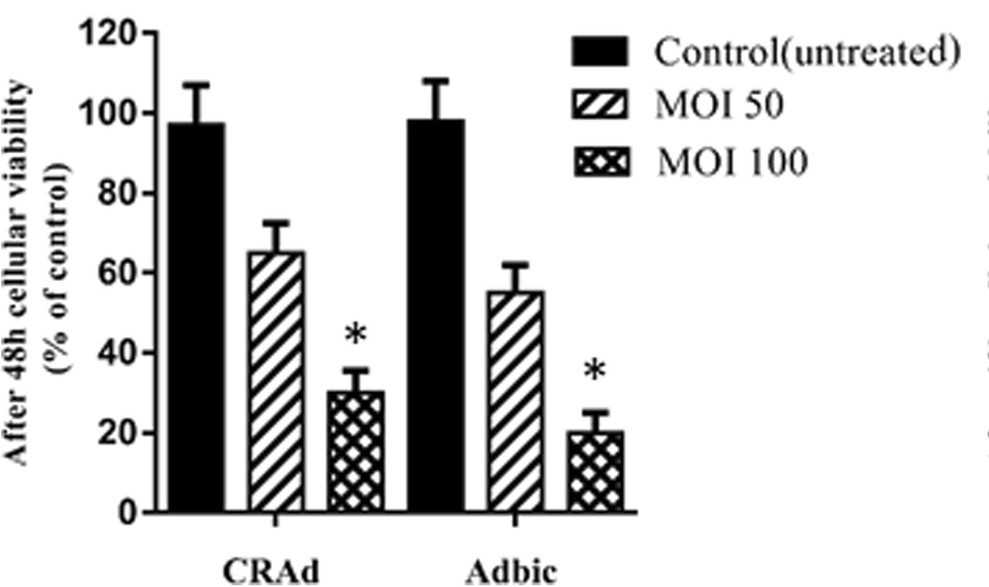 Fig.3 Both adenoviruses CRAd and Adbic can inhibit tumor cell activity in vitro.2,3
Fig.3 Both adenoviruses CRAd and Adbic can inhibit tumor cell activity in vitro.2,3
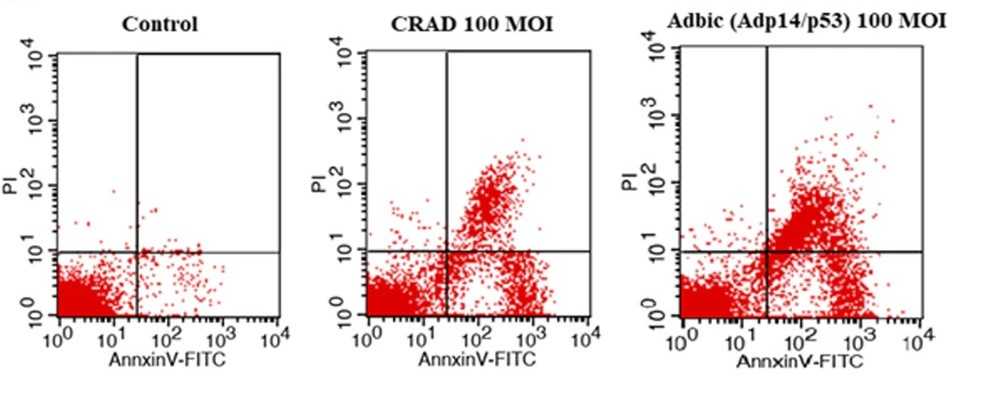 Fig.4 Adenovirus vectors induce apoptosis of tumor cells.2,3
Fig.4 Adenovirus vectors induce apoptosis of tumor cells.2,3
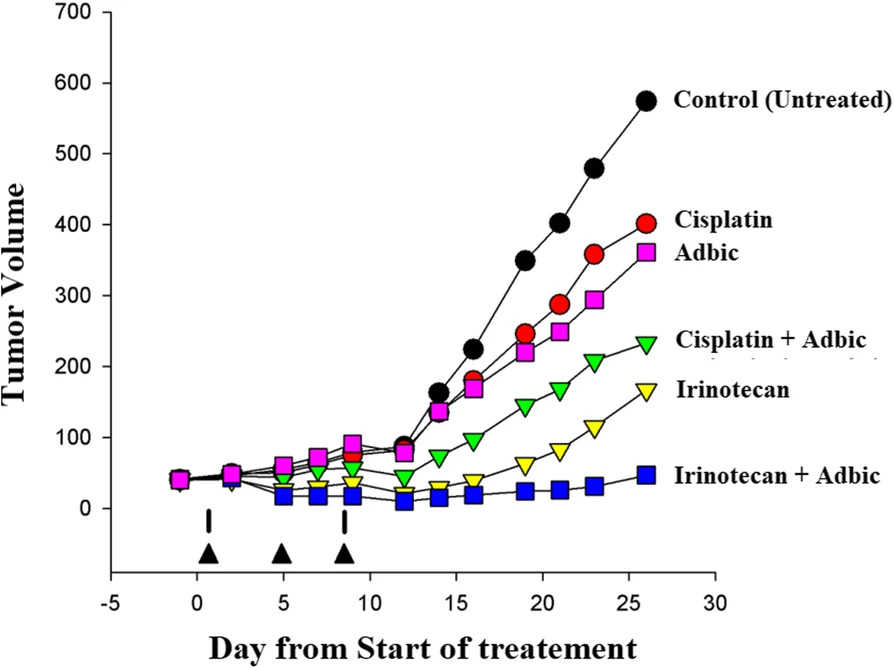 Fig.5 The adenoviral vector inhibited tumor growth in tumor-bearing mice.2,3
Fig.5 The adenoviral vector inhibited tumor growth in tumor-bearing mice.2,3