Custom Lentiviral Vector Production Service
Lentiviral Vector
Lentivirus, a genus of retroviruses, revolutionized gene delivery due to its ability to infect both dividing and non-dividing cells, like neurons and hematopoietic stem cells. Derived from HIV research, it packages genetic material into viral particles that reverse-transcribe RNA to DNA, integrating into the host genome for long-term expression. This feature makes it invaluable for basic research and gene therapy, such as treating genetic disorders, engineering CAR-T cells for cancer, and delivering neurotrophic factors for neurodegenerative diseases.
Creative Biolabs' custom lentiviral vector service ensures premium-quality, high-performance vectors through robust, validated production processes.
Fig.1 Diseases most commo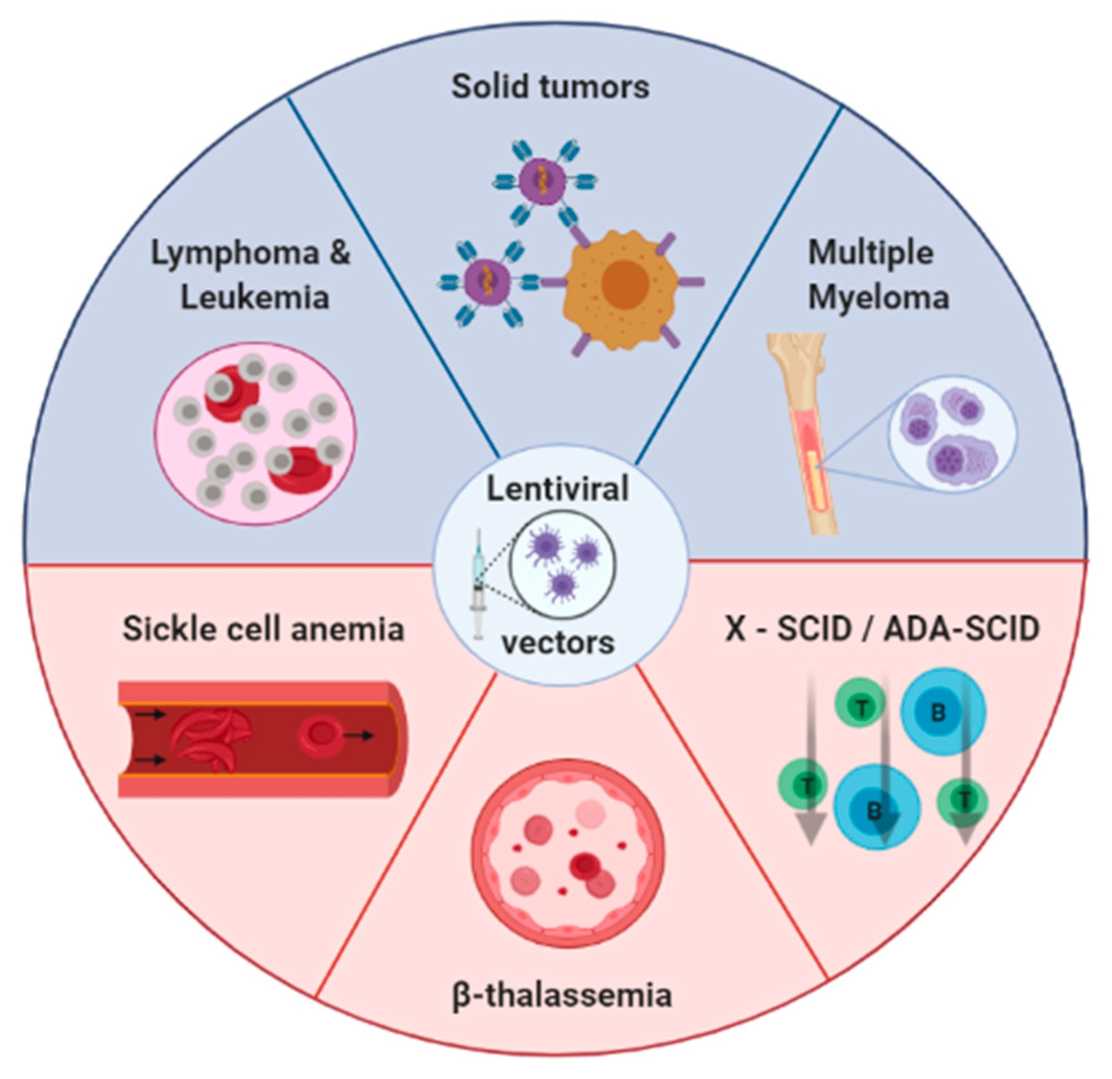 nly treated with lentiviral vectors.1,3
nly treated with lentiviral vectors.1,3
Workflow
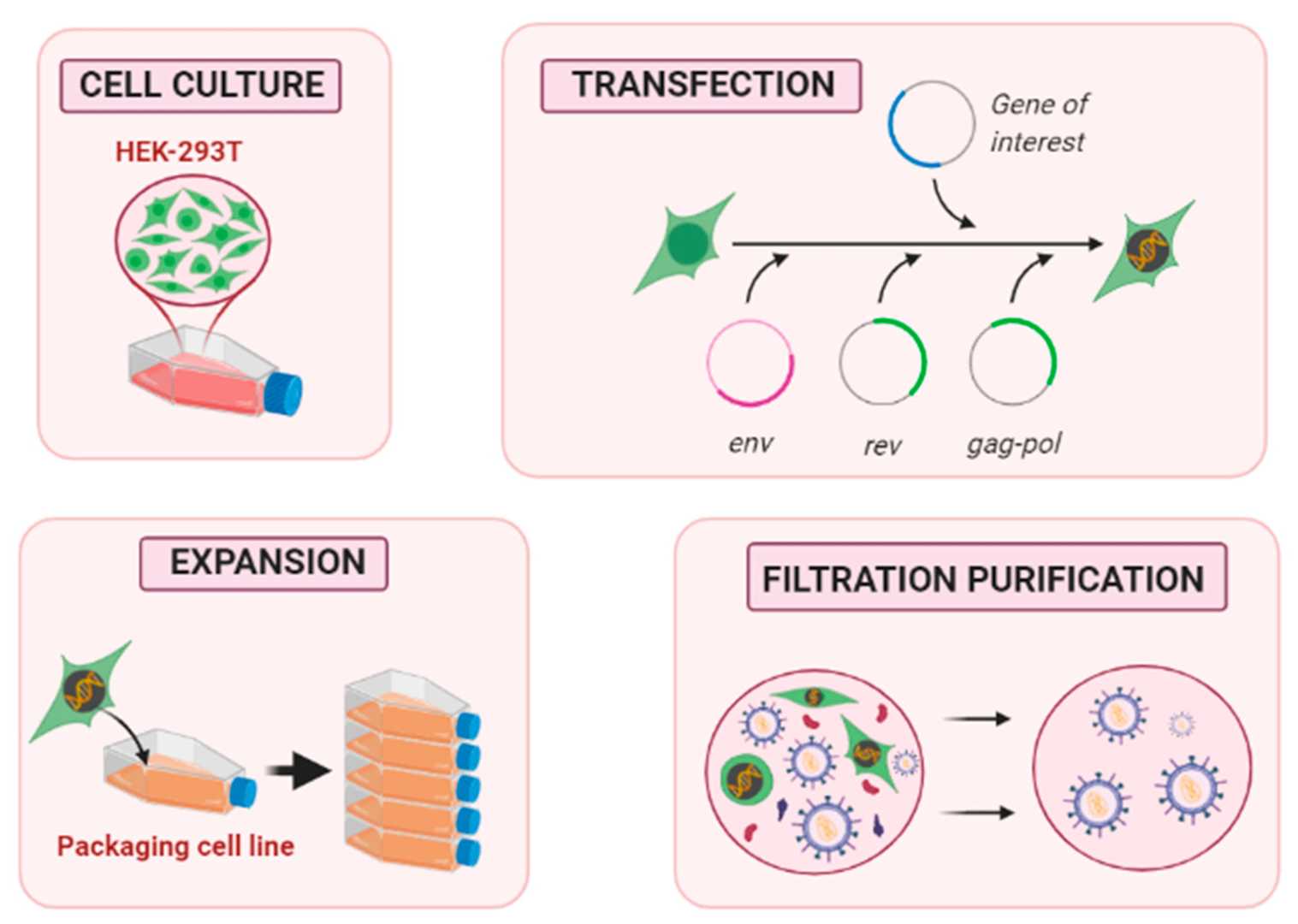 Fig.2 Schematic of the process of lentivirus production, amplification, and purification1,3
Fig.2 Schematic of the process of lentivirus production, amplification, and purification1,3
-
Production
-
Vector Design and Gene Synthesis
Our experts design optimal lentiviral constructs with your gene and regulatory elements. Gene synthesis ensures sequence accuracy and codon-optimized mammalian expression. -
Lentiviral Plasmid Construction
The gene is subcloned into our third-generation backbones, ensuring correct orientation and expression cassette integrity for high-performance vectors. -
Lentivirus Packaging
Constructed plasmids and helper plasmids are co-transfected into producer cells, generating replication-defective viral particles with your genetic payload.
-
Vector Design and Gene Synthesis
-
Purification
- Purification involves clarifying to remove debris, concentrating via ultracentrifugation or tangential flow filtration, and chromatographic steps to eliminate host proteins, nucleic acids, and contaminants. The process yields highly concentrated, pure viral vectors to minimize toxicity and immunogenicity.
-
Quality Control
- Viral Titer Determination
- Purity Assessment
- Sterility Testing
- Endotoxin Testing
- Replication Competent Lentivirus (RCL) Assay
- Functional Validation
Why Choose Us?
Creative Biolabs' dedication to scientific precision, cutting-edge technology, and client-driven outcomes distinguishes us, guaranteeing you receive vectors with consistent, high-performance reliability.
Unmatched Expertise
With 20 years of experience, our scientists possess deep knowledge in lentiviral biology, vector design, and gene delivery, ensuring optimal strategies for even the most challenging projects.
Cutting-Edge Technology
We utilize advanced third-generation lentivirus technology, which provides enhanced biosafety features and superior transduction efficiency compared to earlier generations.
Broad Host Range & Stable Expression
Our vectors are engineered for broad tropism, enabling efficient gene transfer into a wide array of mammalian cells, including critical non-dividing cell types, and ensuring stable, long-term transgene expression.
High Titer and Purity
Our optimized production and purification protocols consistently yield high and ultra-high titer lentiviral vectors with exceptional purity, crucial for sensitive in vitro and in vivo applications.
Comprehensive Quality Control
Every batch undergoes stringent quality control, including titer determination, sterility testing, and functional validation, guaranteeing the reliability and reproducibility of your results.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
Case Study
| Efficiency of Transfection | |
|---|---|
|
|
|
| Concentration of virus | Virus screening |
|
|
|
| The Expression of GFP | |
|
|
|
FAQs
Q: How to overcome immune clearance of lentiviral vectors to achieve long-term expression in vivo?
A: We employ a three-pronged strategy:
- Envelope engineering with CD47 signal peptide to extend blood circulation half-life (3.2x vs. traditional vectors).
- Immunosuppressive conditioning via short-term low-dose cyclosporine to blunt innate immunity without compromising transduction.
- Tissue-specific promoters (e.g., GFAP) for neuro-targeted expression, minimizing off-tissue immunogenicity.
Q: For large-size genes (>7kb), how to overcome the limitations of lentivirus packaging and ensure infectivity?
A: Through "piecemeal delivery-in-situ assembly" technology, where the gene is split into two fragments packaged in separate viruses, allowing intein-mediated protein splicing to reassemble the full protein in target cells. Coupled with ultracentrifugation concentration (100,000g×4h), this yields a 109 TU/mL gene vector with 2.5-fold higher infection efficiency than traditional methods.
Q: How to detect and control the proportion of empty capsids in lentiviral vectors to ensure an effective dose?
A: We employ a three-step QC system: ion exchange HPLC to quantify empty/full capsid ratios (ensuring <15% empty capsids), TEM for direct morphological observation, and qPCR to determine the physical-to-functional titer ratio (P/F ≤10). Through process optimization like low-temperature culture with sodium butyrate, empty capsid proportions are reduced to <5%, significantly boosting functional virus particles per dose.
Q: What storage conditions do you recommend for long-term storage of lentiviral vectors to maintain stability?
A: We recommend storing the stock solution at -80°C immediately after aliquoting to avoid repeated freeze-thaw cycles, which ensures stable storage for 12 months with less than a 1 log decrease in titer. For clinical applications, we provide lyophilized formulations with trehalose protectant, maintaining 90% activity after 6 months of storage at 4°C. During transportation, dry ice (-78°C) or an ultra-low temperature cold chain is used to guarantee no loss of vector activity upon receipt.
Q: How to achieve the targeted delivery of lentiviral vectors to specific tissues?
A: Three strategies can be used to improve targeting: pseudo type modification of APOE as the target; Antibodies/polypeptides (such as RGD peptides) are chemically conjugated to the viral surface through PEG linkers. And tissue-specific promoters loaded with cell-specific expression.
Q: Does your lentivirus production service support customization? Like special promoters or fluorescent markers?
A: Yes, our lentivirus production service fully supports customization to meet your specific needs. We offer a comprehensive range of tailored solutions, including a promoter library with over 20 options like CMV, EF1α, PGK, and inducible systems for synthetic biology designs. For tracking and visualization, we provide various fluorescent markers such as GFP, along with fusion tags like FLAG, HA, or Myc. Additionally, we optimize signal peptide sequences (e.g., IL-2) to enhance secretory protein expression efficiency by 3-fold, and enable precise regulation of dual-gene co-expression through bicistronic designs.
References
- Martínez-Molina, Eduardo, et al. "Large-scale production of lentiviral vectors: current perspectives and challenges." Pharmaceutics 12.11 (2020): 1051. DOI: 10.3390/pharmaceutics12111051
- Kalidasan, V., et al. "A guide in lentiviral vector production for hard-to-transfect cells, using cardiac-derived c-kit expressing cells as a model system." Scientific Reports 11.1 (2021): 19265. DOI: 10.1038/s41598-021-98657-7
- Distributed under Open Access license CC BY 4.0, without modification.

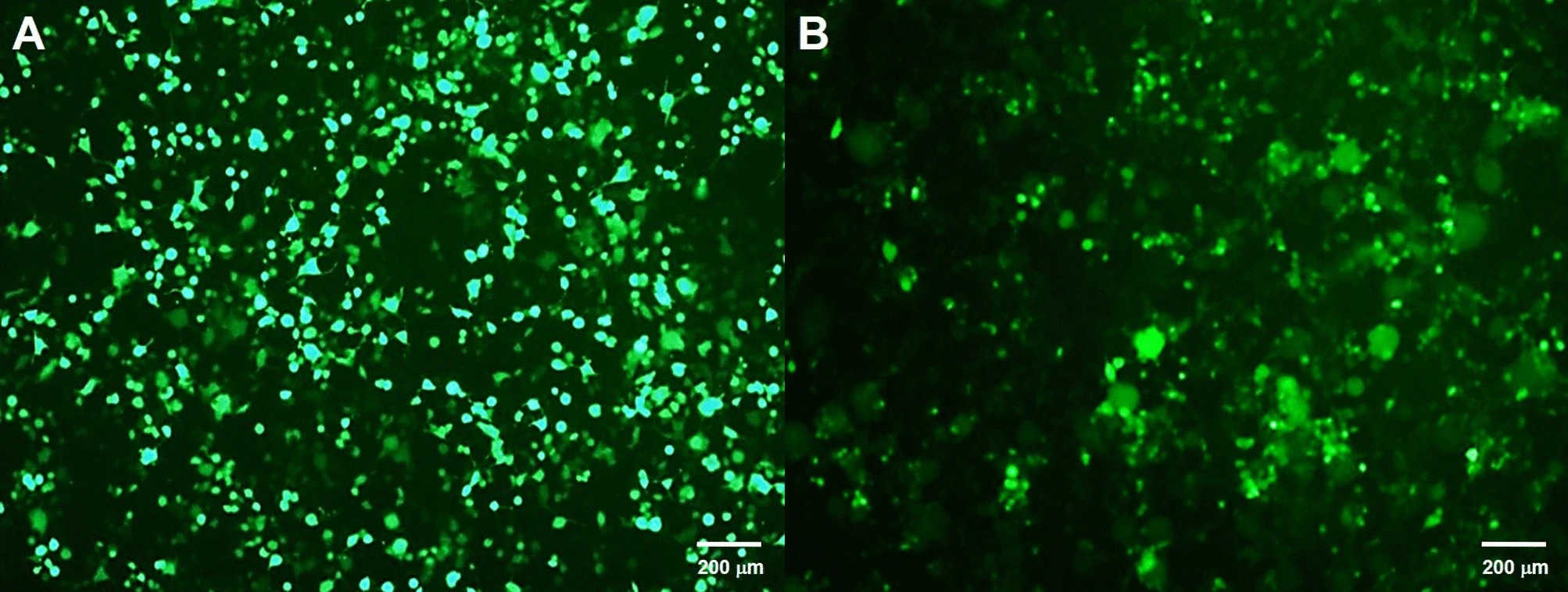 Fig.3 GFP expression in HEK293T cells after 48 hours of infection using the third (A) and second (B) generation lentiviral vectors.2,3
Fig.3 GFP expression in HEK293T cells after 48 hours of infection using the third (A) and second (B) generation lentiviral vectors.2,3
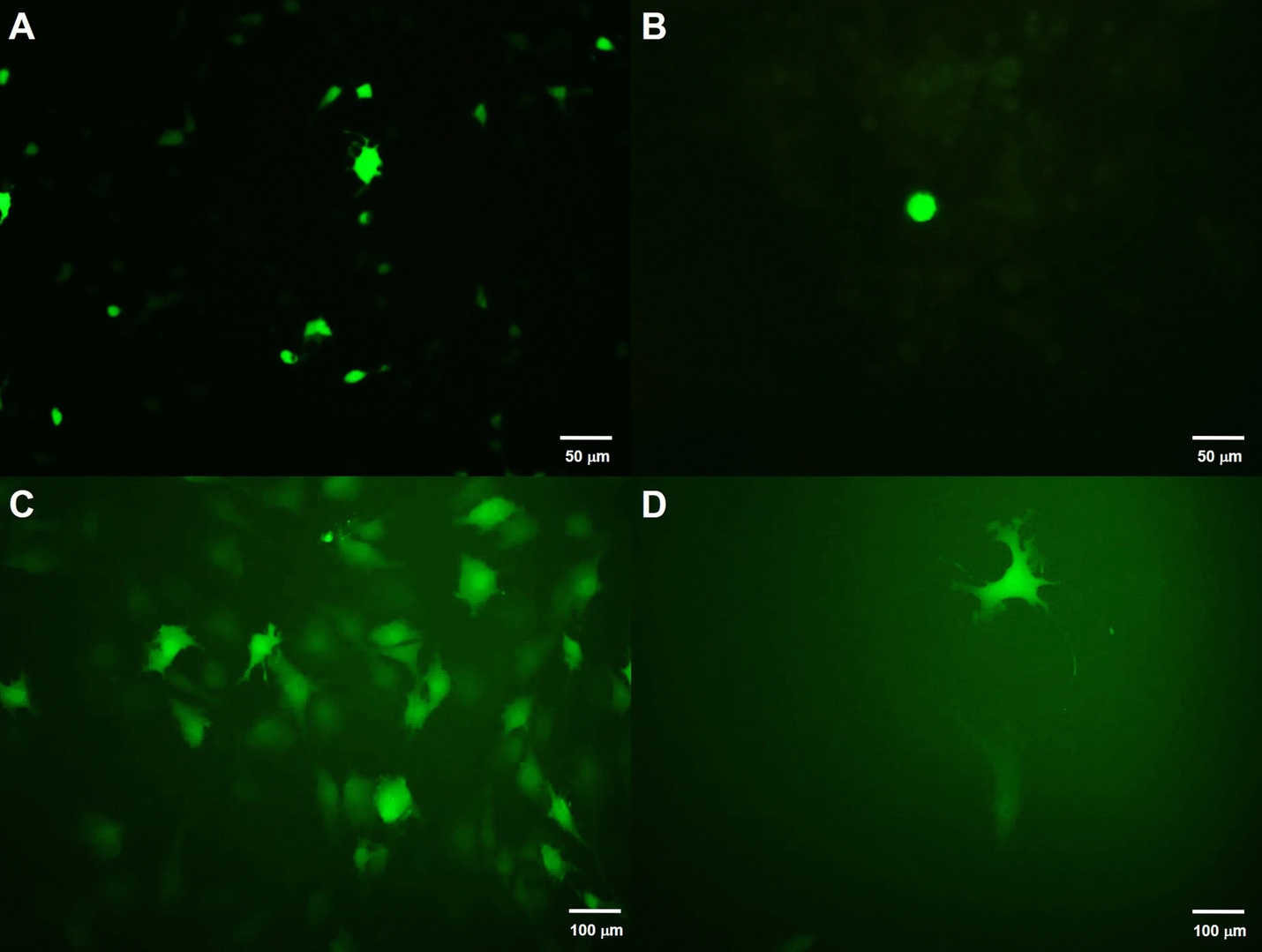 Fig.4 Transfection after virus concentration using different methods, where A: unconcentrated virus; B: concentrated virus; C: concentration by ultracentrifugation; D: 24 hours after common infection.2,3
Fig.4 Transfection after virus concentration using different methods, where A: unconcentrated virus; B: concentrated virus; C: concentration by ultracentrifugation; D: 24 hours after common infection.2,3
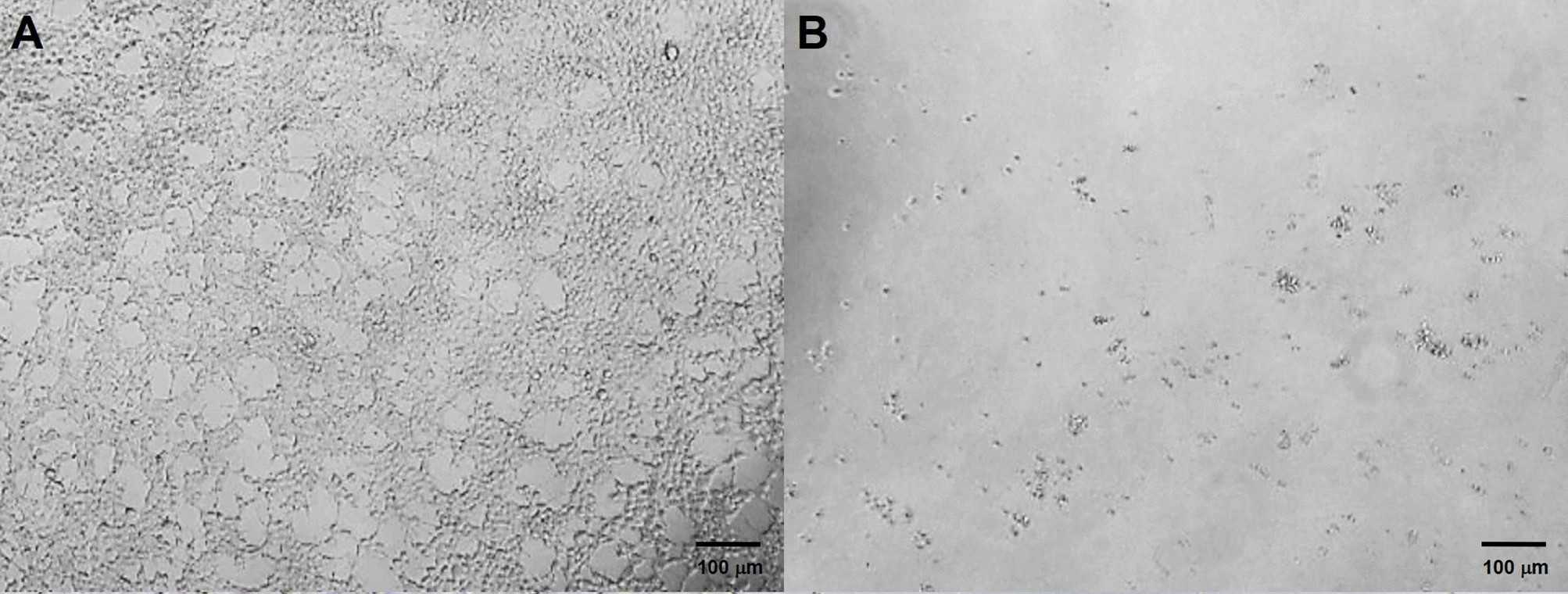 Fig.5 Screening of infected cells with resistance using puromycin.2,3
Fig.5 Screening of infected cells with resistance using puromycin.2,3
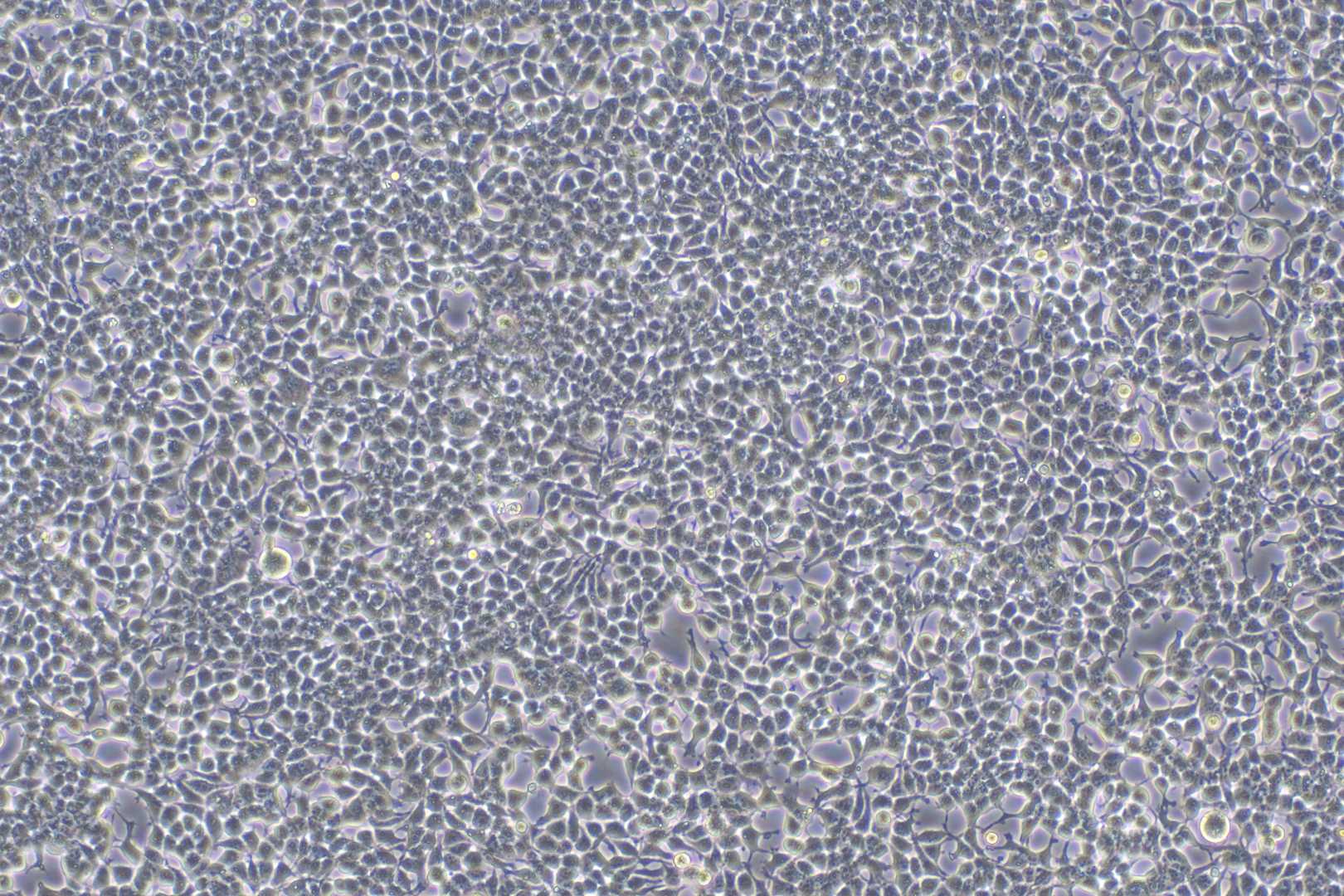 Fig.6 Bright-field plots of HEK293 transfected with LV-GFP.
Fig.6 Bright-field plots of HEK293 transfected with LV-GFP.
 Fig.7 Fluorescence map of HEK293 transfected with LV-GFP.
Fig.7 Fluorescence map of HEK293 transfected with LV-GFP.