Gene Therapy Development for Hematopoietic Disorders
Hematopoietic disorders include those involving plasma protein components of the blood (e.g. hemophilia) and those involving blood cells (e.g. sickle cell disease) and many blood disorders are caused by gene dysfunction. Based largely on biomedicine innovation, gene therapy for hematopoietic disorders has advanced tremendously in the past decades. The approaches by using vector-mediated gene delivery and gene editing have provided clinical benefit to patients with a variety of conditions. As a leading custom service provider, Creative Biolabs has devoted to the development of gene therapy for blood disorders many years. Platforms for the study of protein deficiencies and blood cell diseases have been established in-house by using in vivo gene delivery or ex vivo genetic engineering. We are aiming to accelerate the drug discovery and development process and provide high-quality custom services for our clients.
Featured Techniques for Gene Manipulation
-
Gene Addition
The initial methods for blood disorders gene therapy involve inserting new genes to cells by a variety of viral and non-viral delivery. Generally, in vivo gene therapy is used for the protein disorders while ex vivo gene therapy for the blood cell defects. AAV vectors have been developed to deliver factor VIII or IX expression cassettes for the treatment of hemophilia A or B. Moreover, previous clinical trials have shown that low amounts of an AAV-1 vector given intramuscularly can achieve sub-therapeutic plasma levels of the protein of alpha-1-antitrpsin deficiency. In addition, lentiviral or retroviral vectors are mainly used in ex vivo applications to generate long-term persistence after the cells are re-transplanted by integrating into the cells' genome. Currently, the gene transfer therapy has resulted in several approved therapeutics with more efficacy in earlier stages of development.
-
Gene Editing
Recent progresses in gene editing offer a new modality to achieve permanent genome modification. Gene editing has been applied to genetic hematopoietic disorders, such as ex vivo approaches for protein replacement and in vivo approaches for cell modification. The desired genomes edit can be achieved by utilizing a site-specific nuclease to introduce a DNA double strand break (DSB) at a precise location in the genome. The predominant designer nucleases for gene editing currently under study include transcription activator-like effector nuclease (TALENs) and CRISPR/Cas9. These nucleases can be introduced into cells with various delivery systems for the hematopoietic disorders treatment.
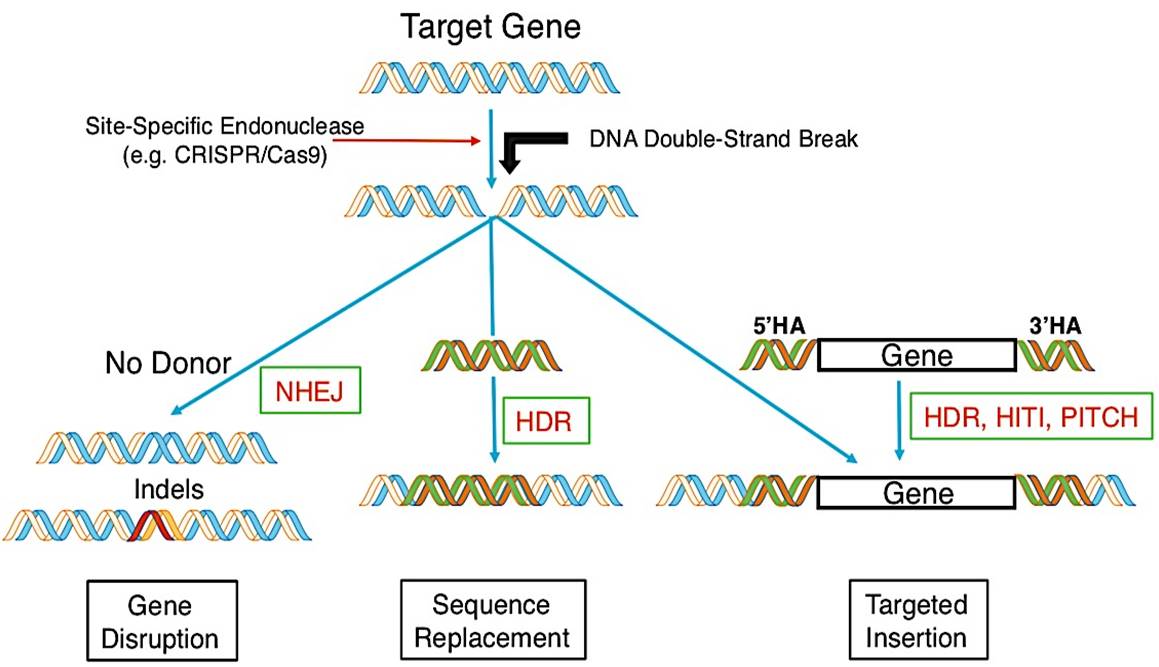 Figure 1. Gene editing (Kohn 2019)
Figure 1. Gene editing (Kohn 2019)
-
Adoptive Cell Transfer (ACT)
Adoptive cell transfer therapy has mostly been performed using hematopoietic stem cells (HSC) and T cells (effector and regulatory). Engineered T cells and regulatory T cells-based therapies may be used to treat hematological disease such as chimeric antigen receptor (CAR)-modified T cell gene therapies , contributing to remarkable successes in the treatment of B-cell lymphomas. Additionally, it has documented that clinical symptoms improved in the majority of beta-thalassemia patients, as well as a few sickle cell patients by the sufficient levels of engrafted gene-corrected HSC. Transduction of gene modificated T cells and HSC may be a promising option for the blood disease therapy.
Creative Biolabs is a leader in the field of gene therapy with great reputation and experience. We focus on the gene therapy development service for our clients all over the world and provide professional and advanced platform to assist in your project. For more detail information, please feel free to contact us.
