Leber Congenital Amaurosis
Leber congenital amaurosis is one of the autosomal recessive hereditary diseases, also called hereditary congenital retinopathy, Alströem-Olsen syndrome, which is the earliest and most serious hereditary retinopathy. The complete loss of binocular cone cell function at birth or within one year after birth will cause the infant congenital blindness. Clinical manifestations include visual loss, neurological deafness, obesity, diabetes, diabetes insipidus, renal insufficiency, hypogonadism, hyperuricemia and hypertriglyceridemia. Currently, scientists use adeno-associated virus (AAV) vectors for gene therapy of Leber congenital amaurosis. AAV based vectors can achieve stable gene transfer with minimal vector related toxicities. Owning comprehensive research background and theoretical foundation in the field of gene therapy, Creative Biolabs can provide you with the most efficient technical services.
Gene Therapy for Leber Congenital Amaurosis
The most common cause of Leber congenital amaurosis is mutations in the gene CEP290 related to the function and quantity of cilia, which accounts for about 25% of the patient population. CEP290 mutations cause a decrease in ciliated cells, resulting in shorter cilia. Delivery of the CEP290 gene to the subretinal by AAV vectors has proven to be a safe and reliable treatment. However, the size of the gene CEP290 (~8kb) exceeds the range that the vector can carry (~4.7kb). How to overcome this obstacle still needs further exploration.
- AAV Vectors Design for Leber Congenital Amaurosis
In recent years, scientists have focused on the use of recombinant AAV vectors for pre-clinical research in gene therapy and have achieved success. These excellent cases provide a solid theoretical foundation and valuable experience for the treatment of clinical diseases. The cDNA encoding miniCEP290 was cloned into the pAAV2 vector plasmid and located between a CMV enhancer/CBA (chicken β-actin) promoter upstream of the internal ribosome entry site (IRES) GFP and β-globin intron. Next, scientists combine the genome of AAV2 with the carrier shell of AAV8 for subsequent research.
Advantage of AAV Vectors
- High biosecurity
- Long-term expression
- Repeatable administration
- Stable physicochemical properties
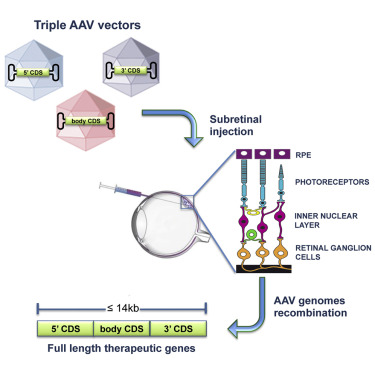 Fig.1 Ocular gene therapy by AAV vectors. (Maddalena, 2017)
Fig.1 Ocular gene therapy by AAV vectors. (Maddalena, 2017)
Taking into account the effects of genetic mutations, AAV vectors are widely used in the treatment of genetic diseases such as choroideremia. Creative Biolabs provides one-stop services such as gene editing, gene insertion, gene coding, and vector preparation. Please contact us in time about detailed information and we are looking forward to your consultation to provide the best quality technical support and testing services for you.
References
- Maddalena, A.; et al. (2017). Triple Vectors Expand AAV Transfer Capacity in the Retina Andrea Maddalena. Molecular Therapy. 26(2): 524-541. Distributed under Open Access license CC BY 4.0, without modification.
