Lentiviral Vector Design Services for Gene Editing
Through decades of development and progress, gene-editing technology has made many breakthroughs in the field of disease treatment. As a lead company committed to human health improvement, Creative Biolabs has developed and licensed a set of advanced genetic therapy CAR-T cell therapy platform to facilitate clients' project progress by offering high-quality gene editing products and services.
Development of Gene Editing
Gene editing, a kind of genome engineering, is a technology to alter (including insertion, deletion or replacement) a specific DNA sequence in the genome of an organism or cell. As early as in the 1970s, scientists successfully introduced exogenous genes into the organisms in a random way. After decades of technique and expertise updating, researchers now can intentionally add, remove or change specific genes at target DNA sites in a rapid and precise manner. Gene editing technology is possibly applied in many fields, such as crops yield increase, pharmaceutical development, diagnosis, etc., particularly in gene therapy.
Currently, gene editing can be achieved by many recognized technologies and methods, including (i) commonly used engineered nucleases like clustered regularly interspaced short palindromic repeats (CRISPR) and transcription activator-like effector nucleases (TALENs); (ii) viral vector systems. Recently, viral vectors as delivery tools are found to exert increasing important actions in gene editing.
Lentivirus Vectors Design for Gene Editing
As known to all, engineered nucleases are the most extensively used approach to perform site-specific gene editing at target locations. Editing genes in vivo to achieve diseases therapy, an appropriate vector system is needed to deliver these nucleases, like TALEN. Compared with non-viral vector delivery, viral vectors exhibit several advantages, among which lentivirus and adenovirus vectors are commonly-used viral vehicles to deliver foreign genes into cells both in vivo and in vitro.
There are numbers of attractive advantages of lentivirus vectors for genes delivery, such as:
- The ability to transduce dividing cells and non-dividing cells, and even differentiated neurons;
- Prominent and efficient gene alteration;
- Lentivirus vector pseudotyping engineering with other viral proteins enables a desired cellular tropism.
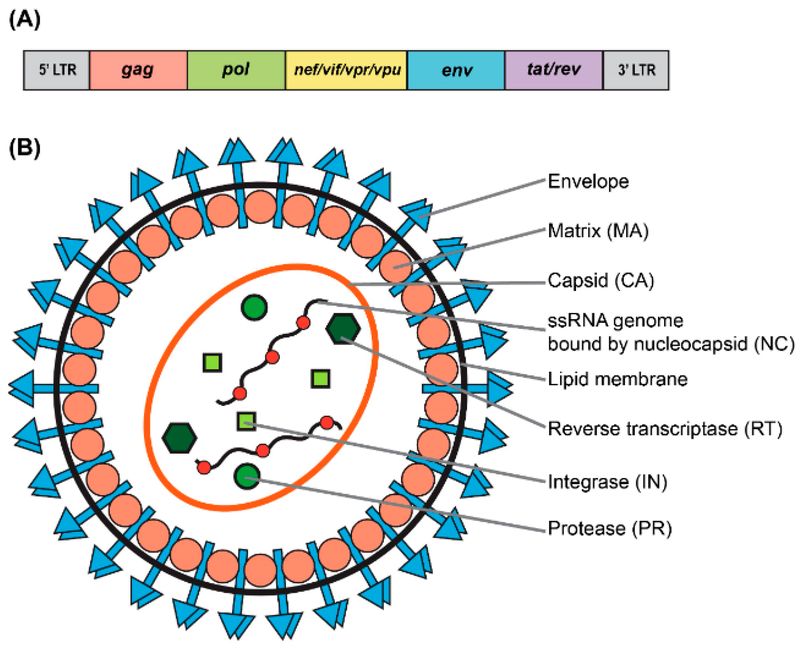 Figure 1. Genomic and structural organization of lentiviral vector. (A) Simplified schematic of the wild-type human immunodeficiency virus type-1 (HIV-1) genome. (B) Particle structure of a lentivirus.1
Figure 1. Genomic and structural organization of lentiviral vector. (A) Simplified schematic of the wild-type human immunodeficiency virus type-1 (HIV-1) genome. (B) Particle structure of a lentivirus.1
Applications of Lentivirus Vectors in Gene Editing
Ex Vivo Gene Therapy
The most common application is the ex vivo modification of cells, a process where a patient's cells are removed, genetically modified with an LV, and then re-infused. A prime example is CAR-T cell therapy, a groundbreaking treatment for certain B-cell malignancies. Lentiviral vectors are used to stably deliver the chimeric antigen receptor (CAR) gene to T cells, reprogramming them to recognize and destroy cancer cells. This approach has led to multiple regulatory approvals and remarkable clinical success. Similarly, LVs are used to correct genetic defects in hematopoietic stem cells (HSCs) for diseases like sickle cell disease and β-thalassemia, allowing the modified cells to differentiate into healthy blood cells with long-term therapeutic effects.
In Vivo Gene Therapy
While more challenging due to the need for precise targeting and avoidance of off-target transduction, LVs are being explored for in vivo delivery, particularly for non-dividing cells like neurons. A recent study demonstrated a novel lentiviral vector (EA1) that achieved effective gene therapy for metachromatic leukodystrophy (MLD) in a mouse model with five times lower vector copy number (VCN), significantly improving safety and efficiency.
Lentiviral Expression Vectors
Lentiviral expression vectors are complex molecular constructs designed to carry and express one or more specific genes in target cells. The design of these vectors is a key determinant of their functionality and is often customized to meet the client's specific research or therapeutic needs.
Single vs. Multiple Expression Cassettes
Vectors can be designed to express a single gene or multiple genes simultaneously. For gene editing, a single vector can be designed to co-express the nuclease and guide RNA (gRNA), a common approach for simple gene knockout experiments.
Reporter Genes and Selectable Markers
The inclusion of a reporter gene (e.g., GFP, RFP) or a selectable marker (e.g., puromycin or gram-negative resistance) is standard practice in lentiviral vector design. Reporter genes facilitate visualization and tracking of transduced cells, while selectable markers enable researchers to select cells that have successfully integrated the vector, enabling the creation of stable cell lines.
ShRNA and Overexpression Vectors
In addition to gene editing, LVs are widely used for gene knockout (using shRNA) and gene overexpression studies. Creative Biolabs offers a range of predesigned and customized vectors for these applications, allowing for rapid and reliable target validation and functional genomics studies.
Core Services at Creative Biolabs
Since the advent of CRISPR, gene editing has made dramatic progress in just a few years. At Creative Biolabs, gene therapy service is our focus as well as flourishing section. Based on the expertise and abundant experience accumulated in the past few years, we provide various lentiviral vector design services for different applications, which include but not limited to:
- Immune Modulation
- Gene Therapy
- Custom Lentiviral Vector
Vector Design: Key Considerations for Gene Editing
Biosafety and Reduced Genotoxicity
A major concern with integrating vectors is the risk of insertional mutagenesis, in which the integrated provirus disrupts or activates nearby oncogenes. While no cases of leukemia have been reported in patients treated with third-generation LVs, our design philosophy is "safety first." We utilize carefully designed SIN LVs and offer integrase-deficient lentiviral vectors (IDLVs) for applications requiring transient gene expression, further reducing the risk of genomic integration and its associated genotoxicity.
Optimizing Gene Expression and Efficiency
The promoter is the engine of the transgene. We offer a comprehensive portfolio of promoters to meet the unique needs of each project:
- Constitutive promoters: Promoters such as the cytomegalovirus (CMV) promoter or the EFS promoter (a version of the EF1α promoter) ensure robust, long-term expression in a variety of cell types, which is critical for correcting genetic defects.
- Inducible promoters: Systems allow precise control of transgene expression, a valuable feature for studying gene function or regulating the dosage of therapeutic proteins. Tissue-specific promoters: For in vivo applications, promoters that drive expression only in specific cell types (e.g., neuronal or liver-specific promoters) are critical for efficacy and minimizing off-target effects.
Our Approach: Precision Engineering Pursuing Excellent Performance
Our scientific team is composed of doctoral level experts who use precise driving methods to ensure the quality and performance of each vector we produce. Our methods include:
- Bioinformatics analysis
- Instantaneous blood transfusion system
- Chromatographic purification
- Functional titration determination
Customer Review

"Working with their Lentiviral Vector Design Services was a game-changer for our gene editing project. Their team tailored vectors to our specific targets, ensuring high transduction efficiency and minimal off-target effects. Deliverables arrived on time, with detailed quality reports that simplified downstream experiments. Their expert support—from initial design tweaks to troubleshooting—saved us valuable time. This collaboration directly boosted our project progress, and we'll definitely partner again."
— Dr. Elena Marquez, Senior Research Scientist
Frequently Asked Questions
Q: How do you ensure the safety of your lentiviral vectors?
A: We use a third-generation packaging system to divide the HIV genome into multiple plasmids that cannot be recombined. We also inserted a self-inactivating (SIN) LTR to prevent the expression of viral genes after integration. In addition, each production batch undergoes validated replication capability slow virus (RCL) testing to ensure the absence of live viruses.
Q: Can lentiviral vectors transduce non-dividing cells?
A: Yes, it is. In fact, this is the key difference between lentiviral vectors and simple retroviruses. Slow virus vectors can transduce dividing and non-dividing cells, including terminally differentiated cells such as neurons and stem cells.
Q: What is the difference between shRNA and miRNA-based silencing, and which does Creative Biolabs recommend?
A: ShRNA typically uses the RNA Pol III promoter (expressed in large constitutive forms), which can cause very strong (potentially cytotoxic) knockouts. On the other hand, miRNA-based silencing utilizes the RNA Pol II promoter, which is more physiologically relevant as it is a natural way of gene regulation. We usually see this method as more stable, less toxic, and more physiologically relevant. We usually sit down with scientists and make recommendations on the best solution for a given project based on the target cell type, the required knockout level, and the possibility of off target effects.
Q: Do you provide support for downstream gene editing validation?
A: Yes, it is. We provide gene editing validation, including T7E1 detection (indel detection), Sanger sequencing (targeted editing), and NGS (non-targeted analysis). We also collaborate with our internal cell biology team to fine tune transduction and editing for your specific cell type.
Q: How do you ensure the absence of replication-competent lentivirus (RCL) in your vectors?
A: We use a four-plasmid system (transfer, Gag/Pol, Rev, VSV-G), which divides viral genes into individual plasmids. This method eliminates the possibility of recombination, thereby eliminating the generation of RCL. In addition, all of our vectors were tested for RCL (gag and env genes) using PCR based detection methods, with a detection limit (LOD) of 1 RCL per 106 TU.
Connect with Us Anytime!
At Creative Biolabs, our deep expertise in lentiviral vector design and state-of-the-art manufacturing platforms make us the ideal partner for your next biomedical breakthrough. Whether you are conducting basic research, developing novel cell therapies, or advancing your project, we offer collaborative scientific partnerships, seamless scalability, and uncompromising quality to accelerate your project from concept to success. If you are interested, please contact us, we can customize our offering to meet your specific project needs.
Reference
- Dong W, Kantor B. Lentiviral vectors for delivery of gene-editing systems based on CRISPR/Cas: current state and perspectives. Viruses, 2021, 13(7): 1288. https://doi.org/10.3390/v13071288 (Distributed under Open Access license CC BY 4.0, without modification.)
