Oligonucleotide-Antibody Conjugation Service
Introduction
Our Oligonucleotide-Antibody Conjugation service accelerates drug discovery with high-quality bioconjugates via advanced chemical modification and purification. Combining antibodies' precise targeting with oligonucleotides' diverse functions, we offer solutions for specific drug delivery, enhanced diagnostic sensitivity, and robust research tools.
[Discover How We Can Help - Request a Consultation]
Oligonucleotide-antibody Conjugation
OAC is a type of biopharmaceutical formed by chemically conjugating oligonucleotides (such as siRNA, miRNA, ASO, or aptamer) with antibodies or their fragments. It combines the targeting specificity of antibodies with the gene regulatory function of oligonucleotides and represents an important advancement in the field of precision medicine.
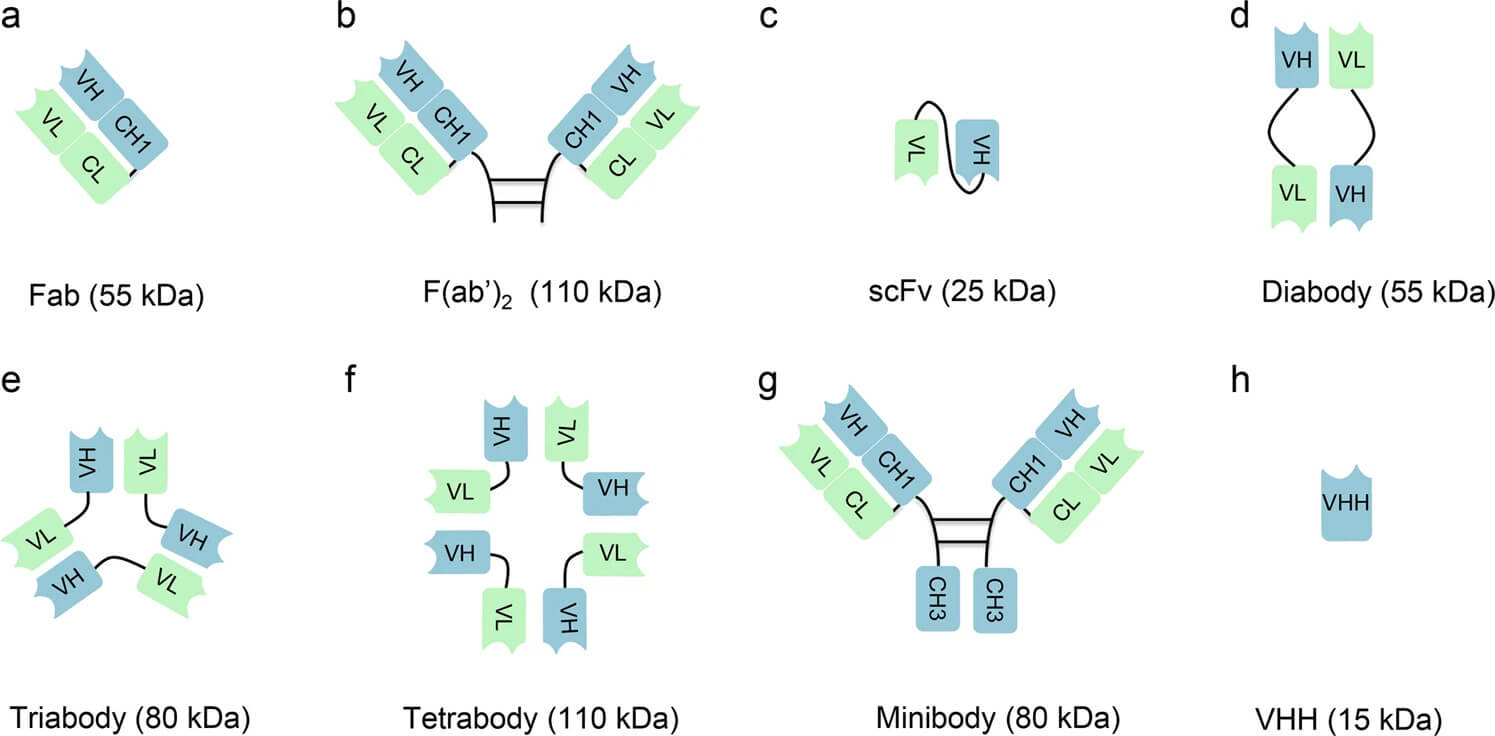 Fig.1 Antibody fragments in different forms and their derivatives.1
Fig.1 Antibody fragments in different forms and their derivatives.1
Classification
According to the types and mechanisms of action of oligonucleotides, OACs are mainly classified as:
- siRNA/ miRNA-antibody conjugate: Guide RNA induces the silencing complex (RISC) to cleave the target mRNA;
- Antisense oligonucleotides (ASO)-antibody conjugate: Inhibits mRNA translation or regulates splicing
- Aptamer-antibody conjugate: Blocks protein activity;
- Immune-stimulating oligonucleotide-antibody conjugate: Activate innate immune signaling pathways.
Characteristics
- Dual targeting: Antibody-mediated cell-specific delivery and gene-level regulation of oligonucleotides.
- Enhance stability: Antibodies protect oligonucleotides from degradation by nucleases and extend their half-life.
- Reduce off-target effects: Antibody-oriented reduction of non-target tissue exposure enhances safety.
- Multi-functional collaboration: It can be designed to simultaneously block protein function (antibodies) and gene expression (oligonucleotides).
- Precise and controllable release: Local activation of the lesion is achieved through cleavable linkers (such as pH-sensitive and enzyme-sensitive linkers).
Mechanism
- Receptor-mediated endocytosis: Antibodies bind to the surface antigens of target cells and enter the cells through endocytosis, releasing oligonucleotides.
- Lysosome escape: The conjugate needs to escape from endosomes/lysosomes to avoid degradation and enter the cytoplasm (such as through membrane fusion or perforated peptide assistance).
- The bystander effect: Some oligonucleotides can diffuse from the initial cell to adjacent cells, expanding their range of action.
Workflow
-
Synthesis
Oligonucleotide-antibody conjugate synthesis involves precise chemical linking, with attention to conjugation chemistry, linker design, and reaction conditions to ensure high yield, homogeneity, and preserved biological activity of both components. Key methods include:
- Ionic Interactions: Uses oligonucleotide's negative charge with antibody-fused/conjugated positive moieties. Simple, flexible but reversible (risk of in vivo instability); polycationic complexes may aid lysosomal escape.
- Avidin-Based Conjugation: Relies on strong biotin-avidin binding, conjugating biotinylated oligonucleotides to avidin-modified antibodies. High stability but requires chemical modification of both components, less general than direct methods.
-
Direct Conjugation: Versatile, preferred method (similar to ADC synthesis). Links chemically modified oligonucleotides to reactive antibody sites.
- Click Chemistry: Reacts azide-modified oligonucleotides with DBCO-modified antibodies (or vice versa). Efficient, biocompatible, widely used.
- Maleimide-Thiol Conjugation: Thiol-modified oligonucleotides react with maleimide-activated antibodies, enabling precise oligonucleotide-to-antibody ratio (OAR) control.
- Enzymatic Conjugation: Uses enzymes (e.g., transglutaminase) for site-specific linking, yielding highly homogeneous conjugates.
- Linker Design: Critical choice of cleavable/non-cleavable linkers impacts oligonucleotide release, in vivo activity, and stability.
- DNA Origami or Hybridization Conjugation: This technique conjugates a single-strand oligonucleotide to an antibody, then hybridizes with a complementary strand. It builds complex nanostructures for carrying multiple therapeutics or as multiplexed diagnostic probes.
-
Purification
Rigorous purification is critical for high-quality AOCs, given conjugation heterogeneity and unreacted materials. Oligonucleotides' distinct properties (larger size, high negative charge) vs. ADC small molecules require specialized purification strategies.
- Ion-Exchange Chromatography (IEX)
- Dialysis and Ultrafiltration
- Size-Exclusion Chromatography (SEC)
-
Quality Control
-
OAR Determination:
- Mass Spectrometry (MS)
- UV-Vis Spectroscopy
- SDS-PAGE and IEX
- PCR-based methods
-
Purity Assessment:
- SDS-PAGE (reducing and non-reducing)
- IEX and SEC
-
Functional Characterization:
- Binding Affinity (ELISA or binding assays)
- Cellular Internalization and Trafficking (confocal microscopy)
- Gene Silencing Activity (qPCR, Western blot, luciferase reporter assays)
- Cytotoxicity (in vitro cytotoxicity assays)
-
Stability Studies:
- Serum Stability
- Storage Stability
-
OAR Determination:
What we can offer
Customized Conjugation Solutions
Tailored linking of oligonucleotides (siRNA, ASO, DNA aptamers) to antibodies with optimized linker chemistry, OAR, and purification, matching therapeutic/diagnostic needs.
Advanced Bioconjugation Chemistries
Expertise in copper-free click chemistry, maleimide-thiol coupling, and enzymatic methods for precise OARs, stable conjugates, and preserved biological activity.
Rigorous Purification and Characterization
Leading IEX for homogeneous conjugates with defined OARs, validated via MS, SDS-PAGE, and UV-Vis for superior purity and integrity.
Enhanced Targeted Delivery & Diagnostic Development
Conjugates optimized for in vivo oligonucleotide stability and targeted uptake (aiding gene therapy) and high-sensitivity diagnostics (e.g., immuno-PCR, CITE-seq).
Experience the Creative Biolabs Advantage - Get a Quote Today
Case Study
| Ion-Exchange Chromatography | Optimize Production Conditions |
|---|---|
|
|
|
| Determination of the Appropriate Reaction Temperature | Immunofluorescence |
|
|
|
FAQs
Q: How does Creative Biolabs ensure the quality and homogeneity of its oligonucleotide-antibody conjugates?
A: Creative Biolabs uses optimized workflows with advanced copper-free click chemistry for conjugation and state-of-the-art ion-exchange chromatography for purification. This yields highly homogeneous conjugates with defined oligonucleotide-to-antibody ratios, minimal impurities, and consistent performance. Each batch undergoes comprehensive QC, including mass spectrometry and functional assays.
Q: Can your service accommodate different types of oligonucleotides and antibodies?
A: Our versatile platform works with diverse oligonucleotides (siRNAs, ASOs, DNA aptamers) and antibody formats (full-length IgGs, fragments, scFvs), with tailored conjugation strategies for unique project needs.
Q: What is the typical turnaround time for an Oligonucleotide-antibody Conjugation project, and what factors might influence it?
A: Our service typically takes 8-16 weeks, varying with antibody/oligonucleotide complexity, conjugation chemistry, desired OAR, and characterization extent. A detailed timeline is provided post-consultation.
Contact Us Today to Begin Your Project
References
- Jin, Shijie, et al. "Emerging new therapeutic antibody derivatives for cancer treatment." Signal transduction and targeted therapy 7.1 (2022): 39. DOI: 10.1038/s41392-021-00868-x. Distributed under Open Access license CC BY 4.0, without modification.
- Wiener, Julius, et al. "Preparation of single-and double-oligonucleotide antibody conjugates and their application for protein analytics." Scientific reports 10.1 (2020): 1457. DOI: 10.1038/s41598-020-58238-6. Distributed under Open Access license CC BY 4.0, the figures were cropped.

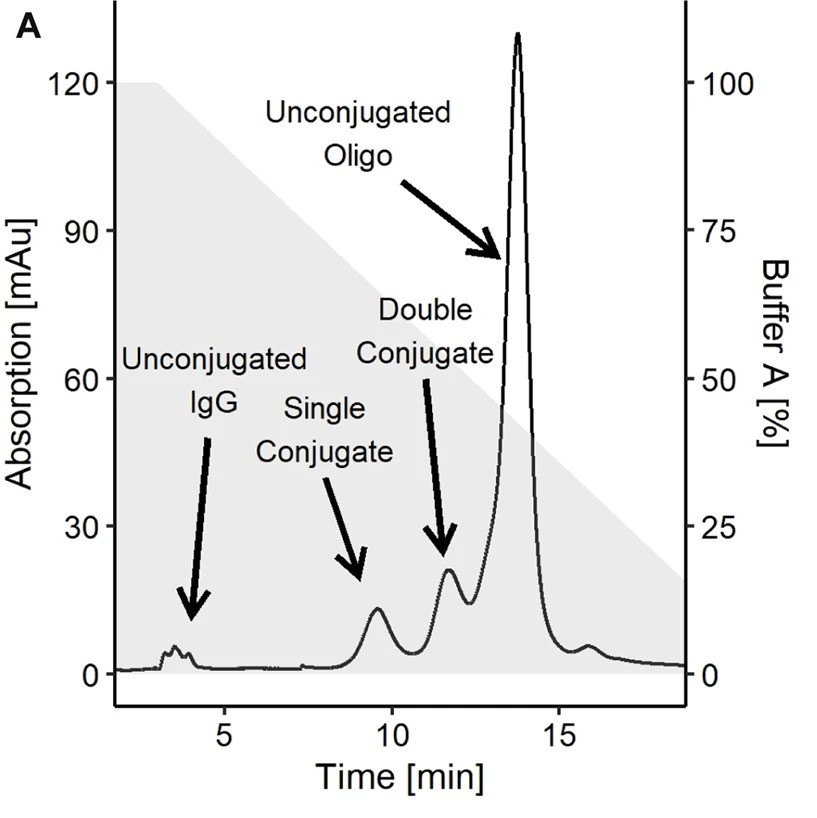 Fig.2 A typical IEX chromatogram of antibody-oligonucleotides obtained using an anion exchange column.2
Fig.2 A typical IEX chromatogram of antibody-oligonucleotides obtained using an anion exchange column.2
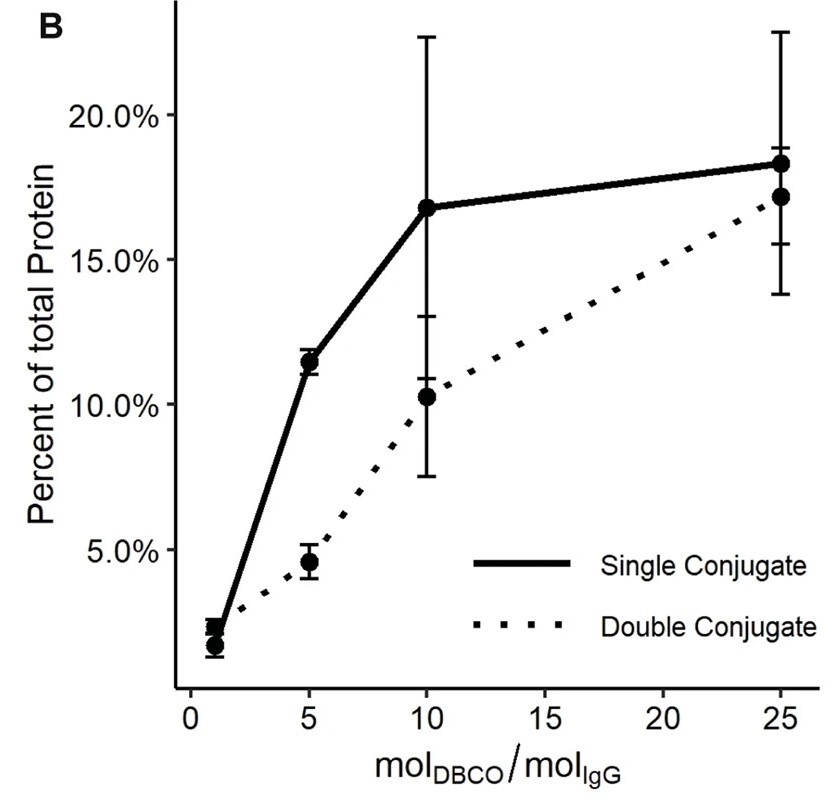 Fig.3 The relationship between the antibody-oligonucleotide click conjugate efficiency and the molar ratio of DBCO to antibody used in the functionalization reaction.2
Fig.3 The relationship between the antibody-oligonucleotide click conjugate efficiency and the molar ratio of DBCO to antibody used in the functionalization reaction.2
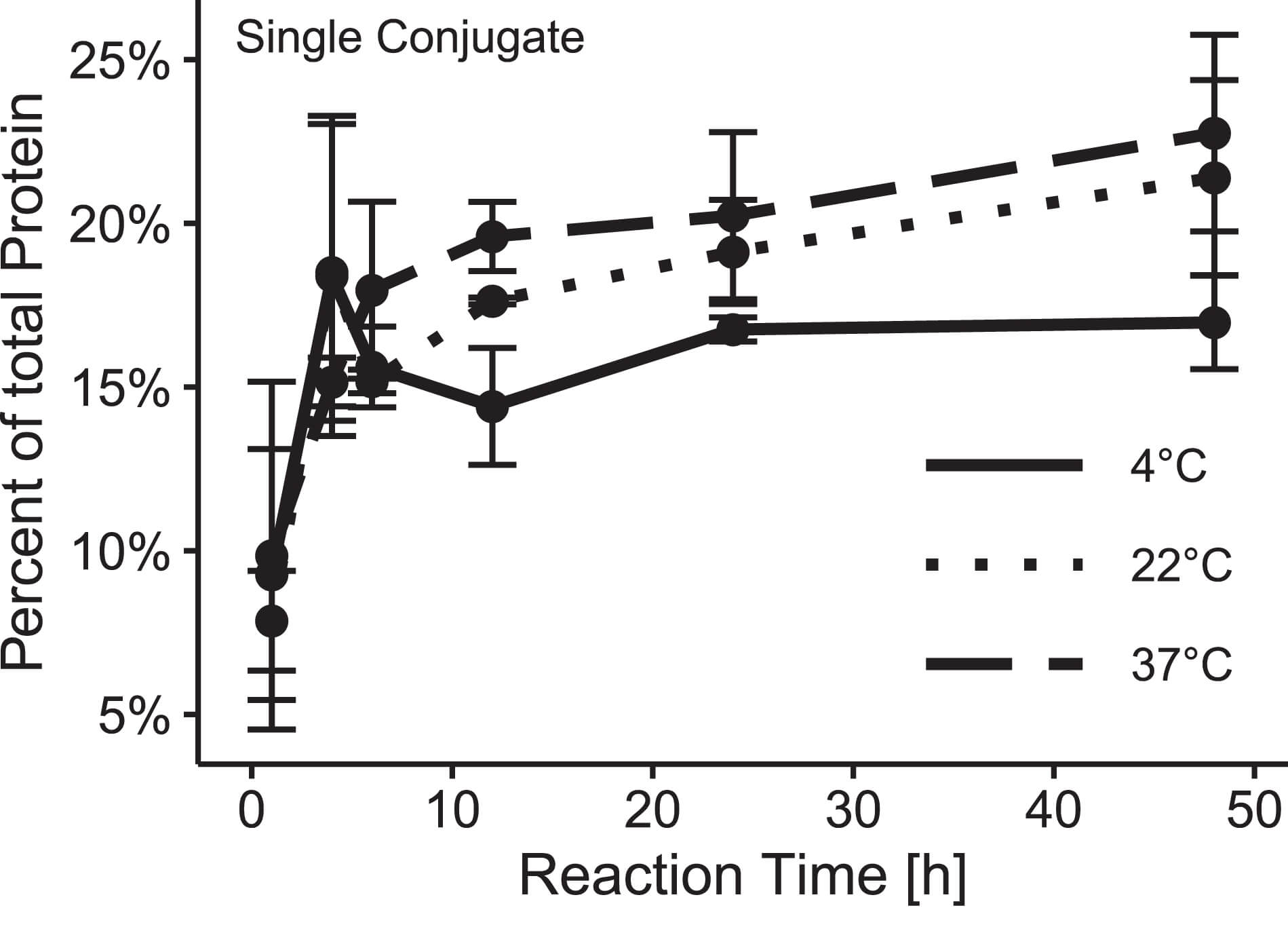 Fig.4 Temperature dependence of oligonucleotide/antibody click-couple linkage mechanics.2
Fig.4 Temperature dependence of oligonucleotide/antibody click-couple linkage mechanics.2
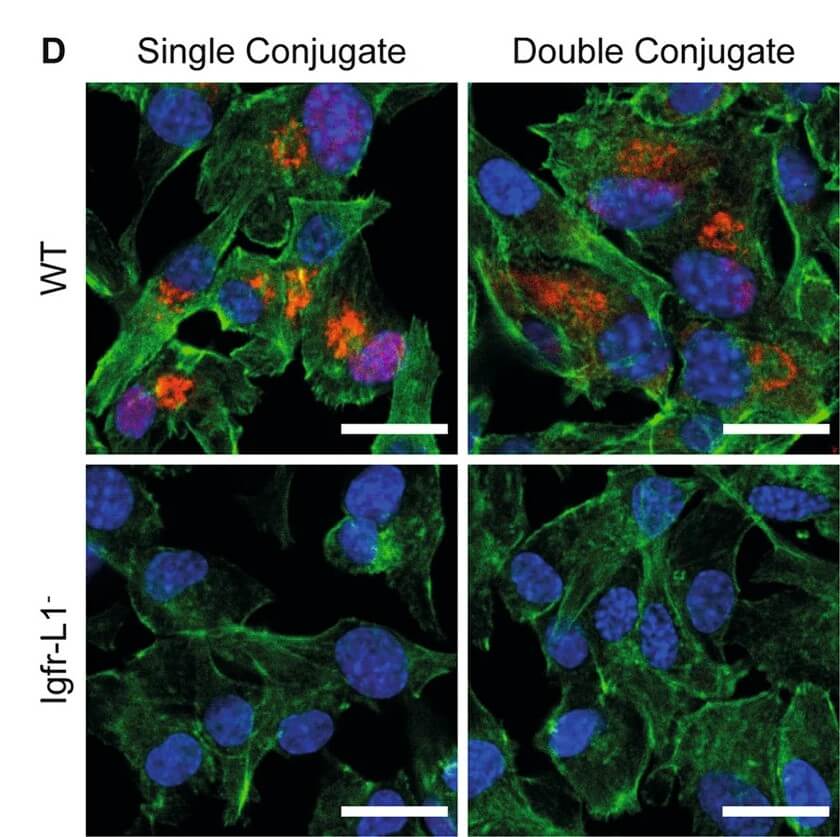 Fig.5 IF images of oligonucleotide-conjugated antibodies in wild-type and target gene knockout cells.2
Fig.5 IF images of oligonucleotide-conjugated antibodies in wild-type and target gene knockout cells.2