Phosphorylation Modification Service
Introduction
Phosphorylation-based modifications in oligonucleotides, like phosphorothioate (PS) or phosphorodiamidate morpholino (PMO), enhance nuclease resistance and cellular uptake, overcoming degradation and permeability issues. They boost stability, efficacy, and delivery. With years of experience, we offer custom synthesis, analytical testing services, and next-gen conjugate development, accelerating oligonucleotide-based drug discovery through advanced chemistry and strict quality control.
[Contact Our Experts for Tailored Solutions]
Phosphorylation
In oligonucleotide modification, phosphorylation refers to the addition of phosphate groups (-PO43-) to oligonucleotide backbones or termini, typically at hydroxyl or amine groups. This process mimics natural post-translational modifications, enhancing biocompatibility while introducing specific functionalities. Related strategies primarily involve chemical modification of phosphodiester bonds or the addition of phosphate groups to optimize the stability, targeting, and pharmacokinetics of oligonucleotides.
| Type | Name | Structural Formula | Characteristic |
|---|---|---|---|
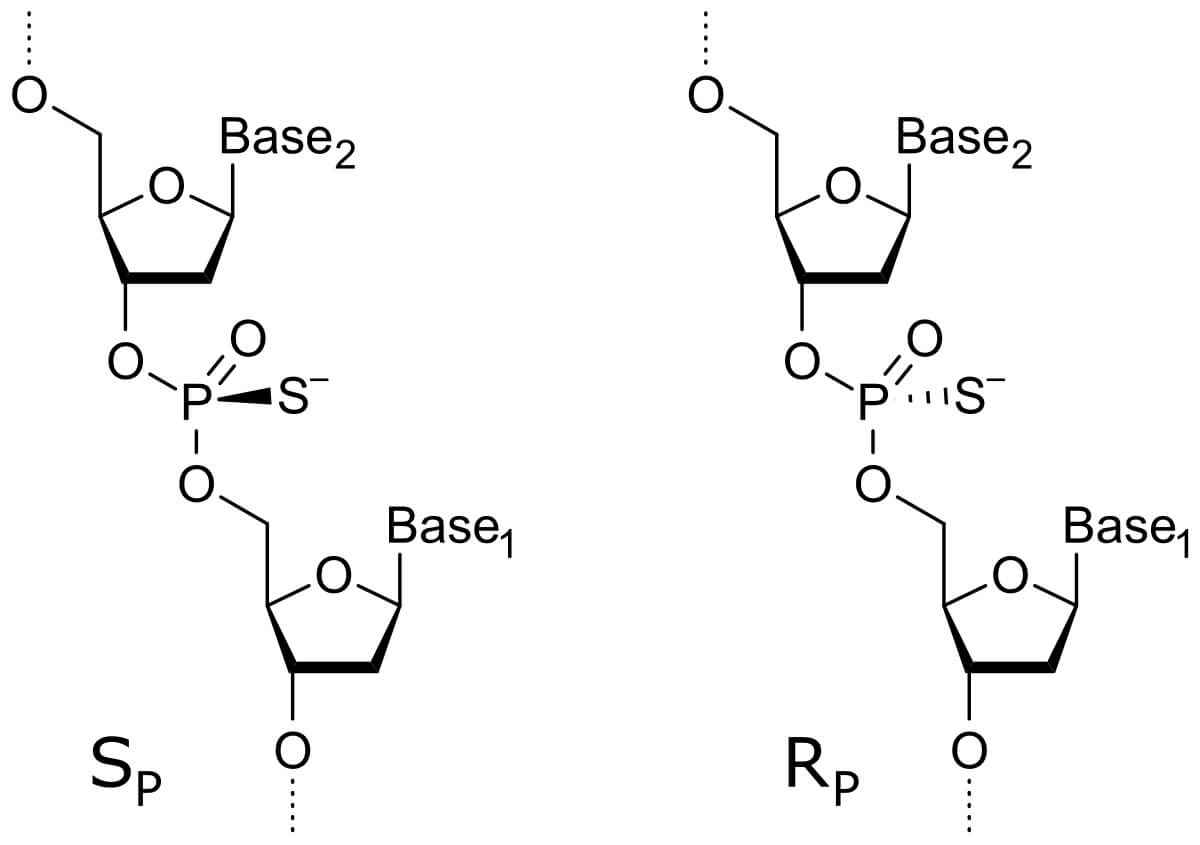
| Backbone Modifications | Phosphorothioate |
|
Replacing the non-bridging oxygen (O) in the phosphodiester bond with sulfur (S) enhances resistance to nucleases and prolongs the half-life in vivo |
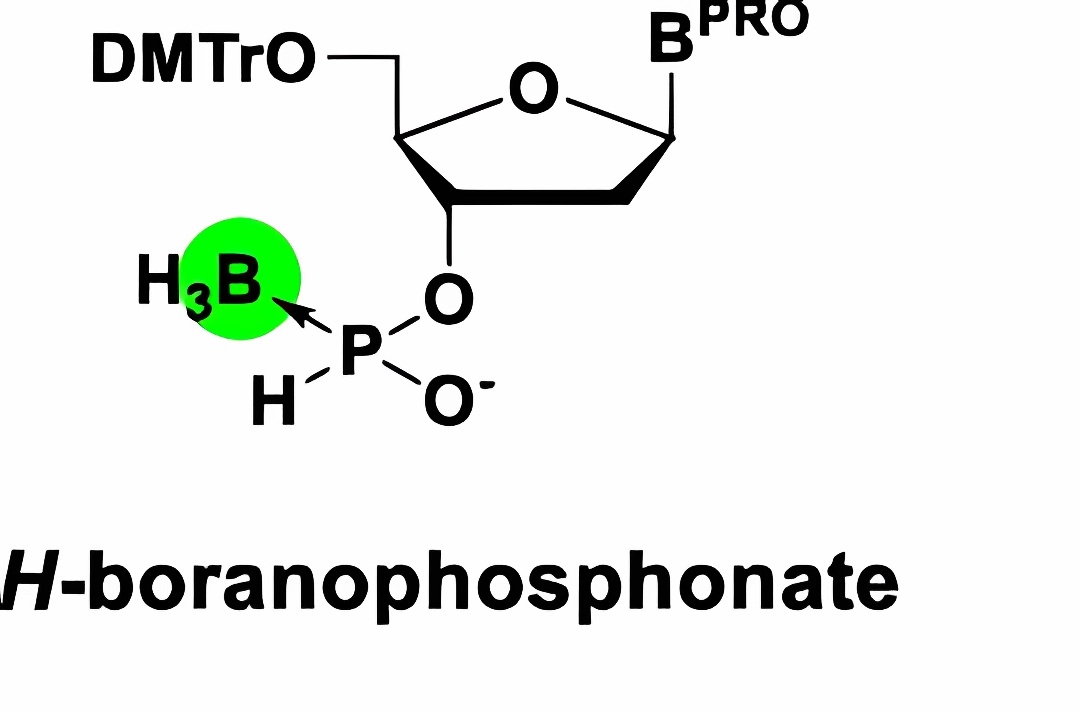
| Boranophosphonate |
|
Replacing non-bridging oxygen with boron (BH3⁻) offers both stability and biocompatibility, improving cellular uptake | |
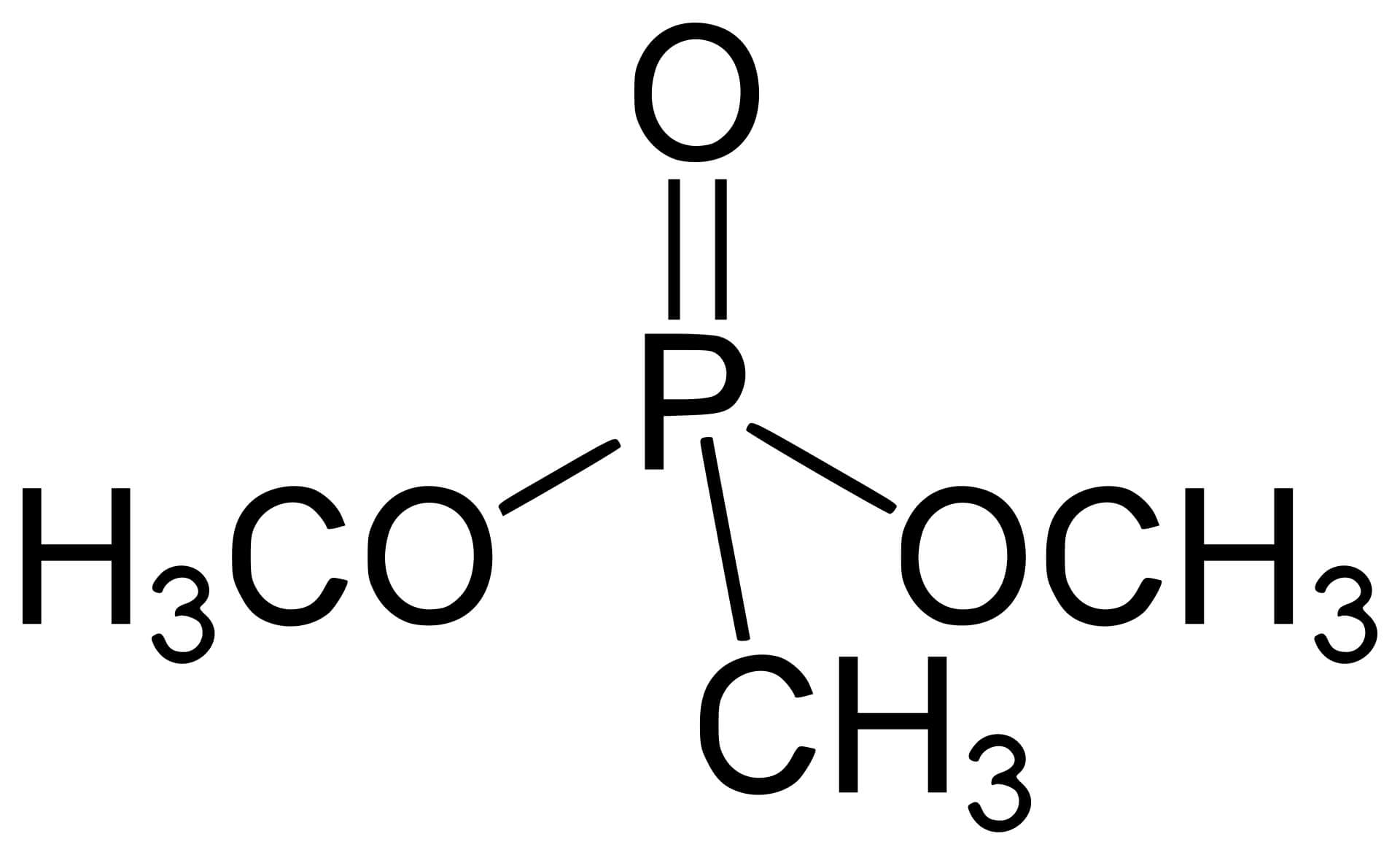
| Methylphosphonate |
|
The non-bridging oxygen is replaced by methyl (CH3), which increases hydrophobicity and enhances the cell membrane penetration ability, but may reduce water solubility. | |
| Terminal Phosphorylation | 5'-phosphorylation | Adding a phosphate group (-PO4) to the 5' end of oligonucleotides is often used in DNA ligation reactions, PCR amplification, or activating RNA interference pathways (such as Dicer enzyme recognition). | |
| 3'-phosphorylation | Protect the 3' end from degradation by exonuclease or use it as a molecular marker for sequencing and diagnosis. | ||
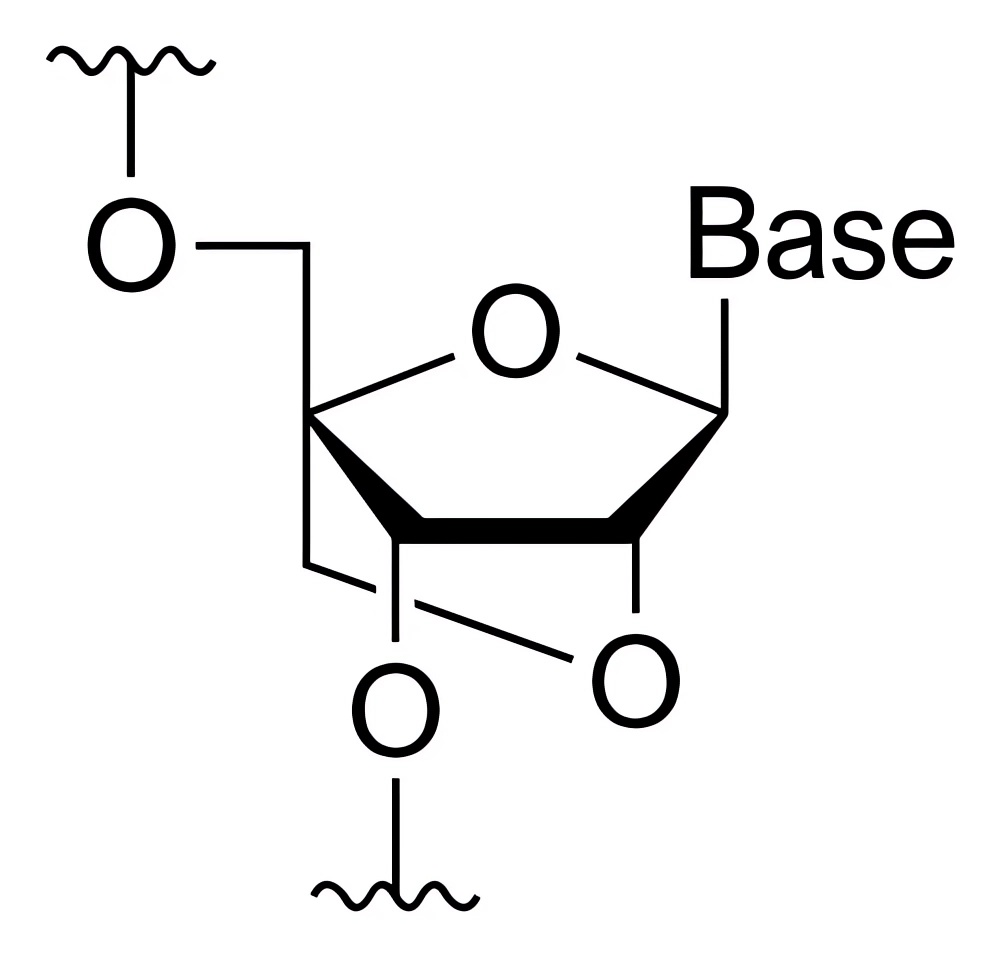
| Functionalized phosphorylated analogues | LNA |
|
The conformation of the sugar ring is locked through the methylene bridge between 2'-O and 4'-C, enhancing the affinity and stability of base pairing. |
| PNA |
|
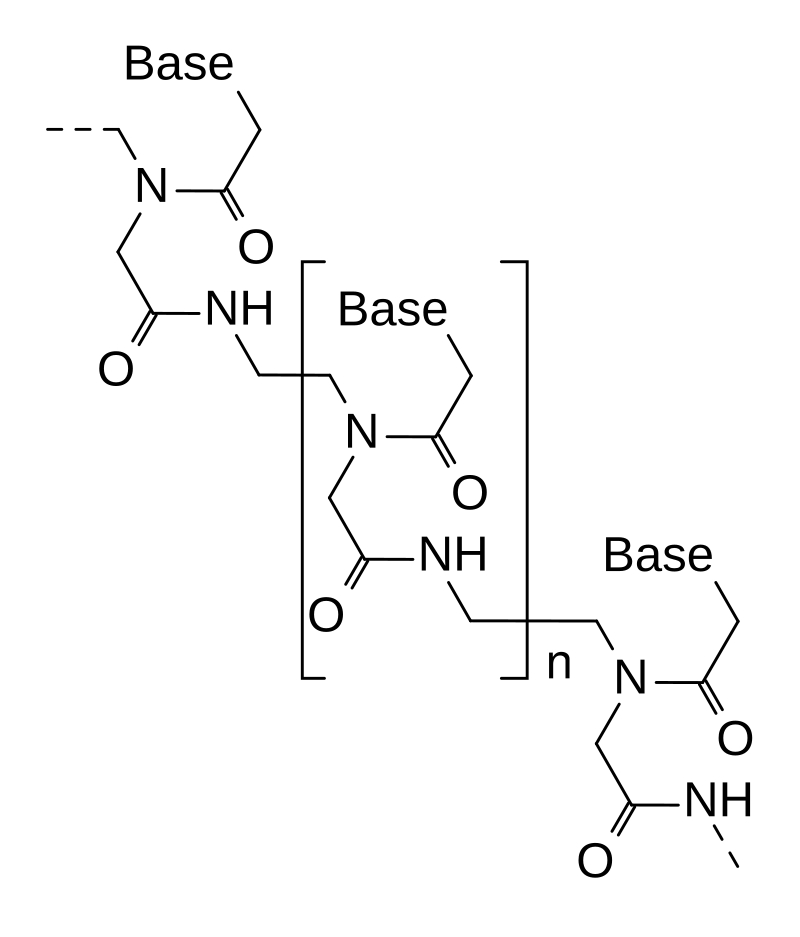
Peptide skeletons are used to replace phosphodiester bonds, which are completely resistant to nucleases and are often used in gene editing tools |
Key Advantages
- Enhanced Stability: Phosphate groups shield oligonucleotides from nucleases extending serum half-life by 3 to 5 times compared to unmodified counterparts
- Improved Targeting: Negative charge modulation optimizes interactions with cell-surface receptors for tissue-specific delivery
- Controlled Immunogenicity: Balanced phosphate density reduces off-target immune activation while maintaining therapeutic activity
Design Considerations
- Site-Specific Placement: Terminal phosphorylation (3'/5') maximizes stability without compromising hybridization efficiency
- Charge Balancing: Excessive phosphate density may reduce cellular internalization empirical optimization ensures ideal charge profiles
- Stereochemical Purity: Chiral control during synthesis minimizes diastereomer-related variability in binding kinetics
Workflow
-
Sequence Design & Consultation
Submit your target sequence and therapeutic goals and our experts will optimize phosphorylation sites using predictive algorithms paired with empirical data These careful tuning balances stability specificity and potency ensuring the modified oligonucleotides align with your research or drug development needs
-
Synthesis & Scale-Up
Our automated solid-phase synthesis systems enable precise insertion of phosphate groups across the oligonucleotide structure Scalable platforms seamlessly accommodate projects from small mg-scale research batches to large kg-level GMP production meeting both early-stage and clinical development requirements
-
Quality Control & Analytics
Rigorous testing covers multiple critical aspects including MS/HPLC analysis to verify molecular weight confirm phosphate incorporation and ensure purity above 98% nuclease resistance assays to validate stability in serum or cytoplasmic lysates and functional testing to measure gene silencing efficiency or target binding affinity
-
Turnaround & Deliverables
The timeline ranges from 8 to 14 weeks depending on sequence complexity and production scale You will receive lyophilized phosphorylated oligonucleotides along with comprehensive datasets detailing purity stability and activity profiles to support your experimental or regulatory needs
Request a Customized Project Timeline
What We Can Offer?
Proprietary Phosphorylation Chemistry
Patented methods achieve >95% modification efficiency with minimal side products, ensuring reliable and high-quality results.
Tailored Design Frameworks
Application-specific optimization for therapeutics, diagnostics, or other, aligning with your unique project goals.
Rapid Turnaround
Expedited options cut timelines by 30% without compromising quality, speeding up your research progress.
Endotoxin-Free Synthesis
Compliant with USP-NF standards, suitable for preclinical and clinical-grade products, meeting strict regulatory requirements.
Integrated Support
From initial design to IND-enabling studies, our team ensures regulatory and technical readiness, supporting your project fully.
[Legare Our Cutting-Edge Oligonucleotide Platform – Schedule a Consultation]
Customer Reviews

FAQs
Q: How does the choice of phosphorylation site (5' vs. 3') impact the function of oligonucleotides in gene editing applications?
A: 5'-phosphorylation often enhances interactions with key gene editing enzymes (e.g., nucleases or ligases), boosting target recognition and cleavage efficiency. 3'-phosphorylation primarily protects against exonuclease degradation but may slightly reduce binding affinity to some editing machinery. We optimize site selection based on your specific gene editing system to balance function and stability.
Q: When using phosphorothioate (PS) modifications in oligonucleotides for in vivo therapeutic delivery, how can we balance enhanced nuclease resistance with minimizing off - target effects and toxicity?
A: PS modifications indeed boost nuclease resistance. However, too many PS bonds can lead to non-specific protein binding and potential toxicity. We recommend strategic placement of PS bonds, often at the terminal ends or near nuclease-sensitive regions. Additionally, optimizing the overall oligonucleotide sequence to reduce self-complementarity can minimize off-target effects while maintaining the benefits of PS modification.
Q: What are the best practices for quantifying the degree of phosphorylation in oligonucleotides, especially when dealing with low - level modifications?
A: Mass spectrometry (MS) is a powerful tool for quantifying phosphorylation. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) MS can accurately determine the mass shift due to phosphorylation. For low - level modifications, liquid chromatography-tandem mass spectrometry (LC-MS/MS) can be used to enhance sensitivity. Additionally, phosphor-imaging techniques can be employed for relative quantification. We use a combination of these methods to precisely measure the degree of phosphorylation in your oligonucleotides.
[Contact Our Team for More Information and to Discuss Your Project]
Reference
- Takahashi, Yuhei, Kazuki Sato, and Takeshi Wada. "Solid-phase synthesis of boranophosphate/phosphorothioate/phosphate chimeric oligonucleotides and their potential as antisense oligonucleotides." The Journal of organic chemistry 87.6 (2021): 3895-3909. DOI: 10.1021/acs.joc.1c01812. Distributed under Open Access license CC BY 4.0, take partial screenshots of the picture.