Safety Determination of Lentiviral Vector Service
Creative Biolabs provides a comprehensive suite of lentiviral viral vector services, including vector engineering, preclinical process development, scale-up production, and analytical development. Our goal is to promote viral vector-based gene therapy to clinical trials for a wide range of disease treatments. We have built a team of talented and motivated scientists and technicians to pursue our commitment.
Lentiviral Vectors Introduction
Lentiviral vectors, a number of gene delivery systems, are derived from lentiviruses that play an important role in transferring targeted genes into specific cells in mammals. Scientists suggest that lentiviral vectors have met with great success, and many laboratories or companies have focused on designing suitable lentiviral vectors to investigate their potential in a wide spectrum of gene therapy applications. In general, lentiviral vectors are designed by replacing the genome of viruses with several marker genes or a series of targeted genes, and viral cis-acting sequences are linked to an expression regulatory domain of transgene. Meanwhile, pilot studies have evaluated the performance of different kinds of lentiviral vectors in most target tissues, including liver, breast, lung, and heart. Furthermore, many advances are also made in vector assessment that is dedicated to improving the safety and effectiveness of lentiviral vectors in gene transfer. Of note, a wide variety of preclinical models, such as animal models and disease models, have been established to reveal the therapeutic potential of novel lentiviral vectors.
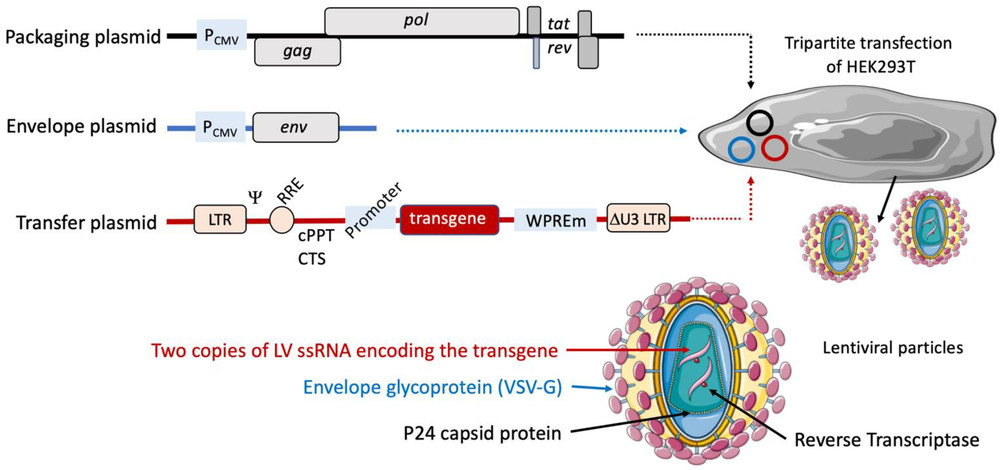 Figure 1. Lentiviral plasmids and the principle of production of lentiviral vectors.1
Figure 1. Lentiviral plasmids and the principle of production of lentiviral vectors.1
Mulligan Lentiviral Vector
The Mulligan lentiviral vector system is named after its developer, representing a significant advancement in the evolution of lentiviral vector technology, with a particular emphasis on enhanced security. The system is built on the third-generation lentiviral platform, incorporating additional modifications aimed at further reducing potential risks associated with vector integration while maintaining high transduction efficiency. The core innovation lies in the strategic separation of viral components across multiple packaging plasmids, minimizing the possibility of homologous recombination that may lead to replication lentiviral virus (RCL).
Key safety features characteristic of the Mulligan system include:
- Enhanced SIN configuration
- Codon optimization
- Tissue-specific promoters
- Advanced envelope pseudotyping
Types and of Lentiviral Vectors
Lentiviral vectors can be classified based on their integration ability and design features, and each vector has different safety considerations and therapeutic applications.
Table 1 Comparison of Lentiviral Vector Types and Safety Features
| Vector Type | Integration Capability | Primary Safety Features | Ideal Applications |
|---|---|---|---|
| Integrating LV | Stable genomic integration | SIN LTR designs, codon-optimized packaging genes, tissue-specific promoters | Long-term gene correction (e.g., hemoglobinopathies), stable cell line generation |
| Integrase-Deficient LV (IDLV) | Episomal persistence only | Mutated integrase enzyme (e.g., D64V), eliminated integration risk | Transient expression, vaccine development, CRISPR/Cas9 gene editing (when integration is undesirable) |
| Bi-cistronic LV | Stable integration | Dual gene expression from single promoter, IRES or 2A elements, reduced need for multiple infections | Co-expression of therapeutic and reporter genes, complex genetic circuits |
| Tissue-Specific LV | Stable integration | Cell-specific promoters (e.g., β-globin LCR), restricted transgene expression | Targeted therapy (e.g., erythroid-specific expression for hemoglobin disorders ), reduced off-target effects |
Safety of Lentiviral Vectors
The path to approval for any LV-based product depends on the successful mitigation and control of two key risks: the potential for generating replication-competent virus and the consequences of genomic integration.
1. Eliminating Replication-Competent Lentivirus (RCL)
RCL is a critical safety hazard that occurs when the vector and packaging plasmids recombine during the production process, resulting in the virus being able to replicate and spread uncontrollably in vivo. Modern third-generation LV systems are designed to minimize this risk by splitting the essential viral components (gag, pol, env, rev) into three or four plasmids, thereby requiring multiple, highly improbable recombination events to restore replication competence.
2. Regulatory Requirements
Despite their complex design, regulatory agencies rigorously require RCL testing for every clinical-grade vector batch. The established "gold standard" involves extended cell culture testing (e.g., up to 21 days) combined with highly sensitive and specific methods such as p24 ELISA and RCL-specific quantitative PCR (qPCR). Failure to achieve a zero RCL result is an absolute deterrent for any investigational new drug (IND) application.
Core Services at Creative Biolabs
Gene therapy has been regarded as an alternative approach for a wide collection of disease therapy. As a perfect gene transfer system, lentiviral vectors have been developed and widely used for expressing high levels of the transgene in specific cells. In order to consider clinical applications, lentiviral vectors must meet the strictest safety standards due to their virus natures. As a result, Creative Biolabs has established a panel of assays to evaluate the safety of various kinds of lentiviral vectors, such as HIV-1 based lentivirus vector evaluation assay and non-human lentivirus vectors evaluation assay.
Comprehensive Analytical Testing
| Assay Module | Key Test Items | Scientific Rationale & Importance |
|---|---|---|
| I. Vector Characterization & Potency | Functional Titer (TU/mL): FACS or qPCR. | Determines the biological activity and infectious dose (efficacy and safety). |
| Physical Titer (VP/mL): p24 ELISA or Total RNA qPCR. | Key metric for process control; the TU/VP ratio serves as an indicator of vector quality and purity. | |
| II. Replication Competence Analysis | RCL Assay: Prolonged Culture, p24 ELISA, RCL-Specific qPCR. | The single most critical safety test. Mandatory regulatory requirement to prevent uncontrolled in vivo virus spread. |
| III. Purity & Adventitious Agent Testing | Sterility & Mycoplasma Testing: Culture-based and qPCR. | Mandatory to exclude contamination by common microbial and cell culture agents, preventing patient infection. |
| Endotoxin Assay (LAL Test): | Assesses vector purification efficiency; high endotoxin levels can cause severe systemic inflammatory (pyrogenic) reactions in vivo. | |
| IV. Host Cell Residuals | Host Cell DNA Residuals: HEK293T DNA qPCR. | Quantifies residual producer cell DNA; residual DNA is a safety concern regarding oncogenicity and immunogenicity. |
| Host Cell Protein Residuals: ELISA. | Measures residual non-viral proteins from the production process, confirming purification efficacy and minimizing allergic/immune reactions. |
HIV-1 Platform
In our HIV-1 platform, replication-competent lentivirus (RCL) has been detected by using a positive control. Moreover, the vector titer, the number of vectors, as well as the potential containment of a wide variety of animal hosts have also been assessed. Currently, we have successfully developed a number of detection systems for our clients to estimate the pharmacodynamics and biosafety of lentiviral vectors. As illustrated, a clinically applicable lentiviral vector has been established to assess the biosafety and efficacy of gene transfer into patient-derived hematopoietic stem-cells. The data indicate that this vector shows a beneficial safety profile both in vitro and in vivo.
Features of Our Services
- Unparalleled Scientific Expertise: Our team is comprised of PhD-level scientists with extensive experience in vector biology, gene therapy, and regulatory affairs.
- State-of-the-art Facilities: We operate in a cGMP-compliant environment using the latest analytical instrumentation to ensure the highest quality and reproducibility of our results.
- Comprehensive Services: We provide a one-stop solution, from initial vector design consultation to final regulatory filings.
- Timely and Transparent Communication: We are committed to providing timely updates and transparent data reporting throughout your project.
Customer Review

"We entrusted Creative Biolabs to conduct a pivotal safety assay for our lead lentiviral vector candidate in preclinical trials. Their team's expertise and attention to detail were outstanding. The comprehensive data package they provided was instrumental in enabling our studies. Their methods, especially the advanced integration site analysis, gave us the confidence we needed to move forward. The entire process was seamless, and their scientific support was invaluable."
— Dr. Raj Patel, CEO
Frequently Asked Questions
Q: What is the most important safety concern with lentiviral vectors?
A: The main safety issue previously attributed to the use of lentiviral vectors was the possibility of insertional mutagenesis. Such an event, i.e. inactivation of tumor suppressor genes or activation of oncogenes due to vector integration, could result in a malignant phenotype. The risk of insertional mutagenesis with the use of safety-enhanced vectors has been shown to be much reduced and no evidence of genotoxicity in this context has emerged from the numerous clinical applications with these new vectors whereas such a risk has been recently re-identified in the case of first-generation retroviral vectors.
Q: How do you ensure detection of replication-competent lentivirus (RCL)?
A: Our RCL assay uses a tiered approach, beginning with a highly sensitive co-culture assay using a licensed cell line designed to amplify any potential RCL, followed by PCR detection of specific viral sequences (gag, pol, env). This approach can detect extremely low levels of RCL (with a sensitivity of <1 RCL per 1×10^8 vector particles). We incorporate multiple positive controls to validate assay performance in each run.
Q: What vector copy number (VCN) range is considered safe?
A: The optimal VCN range depends on the specific application, but for most therapeutic uses, it is typically between 1 and 5 copies per cell. Higher copy numbers may increase genotoxicity risk without necessarily providing additional therapeutic benefit. Our safety assessment includes determining the relationship between VCN and therapeutic efficacy to establish a safe therapeutic window for each application.
Q: How long does a comprehensive safety assessment typically take?
A: Depending on the specific testing required, a complete safety analysis, including RCL testing, VCN assay, integration site analysis, and sterility testing, typically takes 4–8 weeks. Due to culture requirements, some tests, such as sterility (14 days) and RCL (3–4 weeks), have fixed durations. We strive to provide rapid service whenever possible for projects with critical timelines.
Q: Can you handle specialized lentiviral vectors, such as integrase-deficient (IDLV) systems?
A: Yes, our safety assay service is fully validated for all lentiviral vector types, including integrase-deficient lentiviral vectors (IDLV). For IDLV, we have modified the integration site analysis to focus on sporadic persistence and provide quantitative data on the percentage of cells maintaining sporadic vector over time, which is critical for understanding the duration of expression.
Q: Can you help us design our vectors for optimal safety?
A: Absolutely. Our expertise extends beyond testing. We offer consulting services to help you design vectors with optimized safety profiles, including selecting the appropriate promoter, safety profile, and therapeutic gene sequence to minimize off-target effects and immunogenicity.
Connect with Us Anytime!
As a reliable CRO company, Creative Biolabs is committed and proud to be part of this rewarding challenge by offering its safety support to the pre-clinical and commercial manufacture of your viral vectors. We are proud to partner with our clients on the journey of bringing novel lentiviral vectors to gene therapy applications. If you are interested in our services, please contact us for more details.
Reference
- Nemirov K, Bourgine M, Anna F, et al. Lentiviral vectors as a vaccine platform against infectious diseases. Pharmaceutics, 2023, 15(3): 846. https://doi.org/10.3390/pharmaceutics15030846 (Distributed under Open Access license CC BY 4.0, without modification.)
