Sonoporation
To date, several gene delivery methods have been developed for treating many serious diseases that conventional drug therapies cannot cure. With the advancement of physical technology, some physical gene delivery methods such as electroporation and sonoporation have been extensively developed and are receiving increasing attention. Among them, sonoporation based on ultrasound and microbubbles has become increasingly popular for systemically targeting drugs and genes due to its simplicity and nontoxicity.
Introduction of Sonoporation
Sonoporation is a method for the introduction of genes into target tissues, which can deliver genes to the corresponding tissue by the combination of ultrasound exposure and subsequent rupture of microbubbles. Sonoporation is to perforate the cell membrane by using ultrasound waves. Ultrasound frequency covers a broad range from 20 kHz to 5 MHz for gases and 500 MHz for liquids and solids, but it should be noted that sonoporation mainly uses ultrasound waves at megahertz frequencies. Ultrasound was first used to transfect mammalian cells in vitro in 1996 and was widely used for gene delivery in the 2000s.
The Principle of Sonoporation
Cell membranes often prevent large molecules such as drugs and genes from entering cells. The mechanical force of focused ultrasound can change the permeability of cell membranes and allow a greater volume of compounds to enter the cell through stable cavitation. This effect, also known as sonoporation, improves the efficacy of drugs and genes in precise areas in the body.
In sonoporation, a high-intensity ultrasound field forms a pore on the cell membrane, and then the exogenous nucleic acid is delivered into the cell. When ultrasound acts on the cell membrane, two physical effects occur, namely thermal effects or non-thermal effects. At low ultrasound intensity, non-thermal effects are produced, including cavitation, mechanical streaming and radiation forces. These effects are used to perform ultrasound-mediated delivery. Cavitation is formed in a liquid containing gaseous bubbles driven by a low-intensity ultrasound. As the intensity of the ultrasound changes in a sine shape, the cavitation bubbles change periodically between the states of compression and rarefaction. When the ultrasound intensity increases instantaneously, the cavitation bubble collapses immediately (Fig.1), which causes shock waves and microjets to perforate the cell membrane.
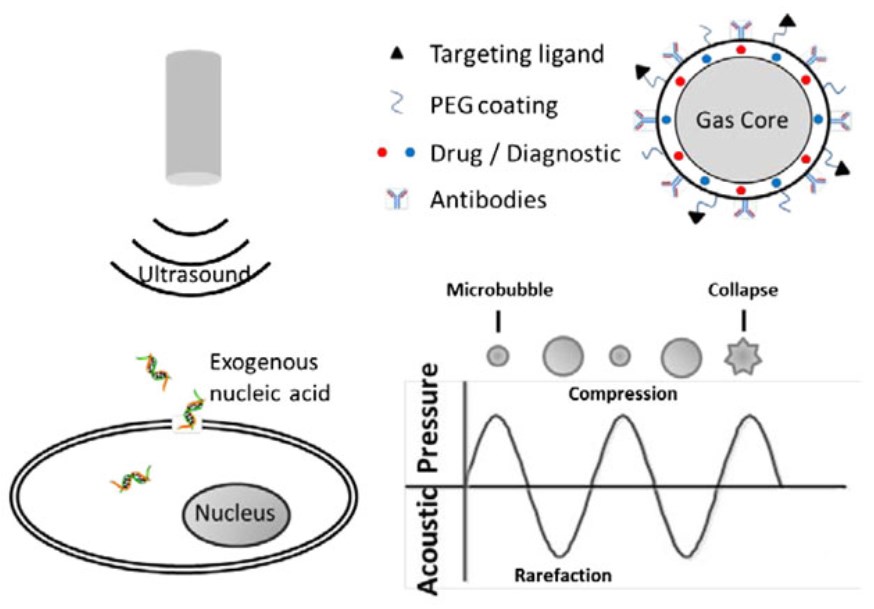 Figure 1. The principle of sonoporation. (Du, 2018)
Figure 1. The principle of sonoporation. (Du, 2018)
In recent years, microbubbles have been used to improve the transfection efficiency of sonoporation. Microbubbles are gas-filled vesicles encapsulated by stabling shell which can be functionalized by drugs, PEG, targeting ligands and antibodies. The presence of microbubbles can reduce the threshold of sonoporation and prompt gene delivery. Shapiro et al. pointed out that the concentration of microbubbles should be controlled within a narrow range to achieve enhanced sonoporation. Too high or too low concentration of microbubbles may lower the transfection efficiency of sonoporation. Recently, researchers have used nanobubbles as an effective contrast agent for sonoporation-mediated gene transfection. In addition, other nanocarriers, such as polymeric micelles, polymeric nanoparticles, nanoemulsions or liposomes, are also used in combination with sonoporation to improve transfection efficiency.
Sonoporation has several advantages:
- It is simple and involves two main steps-preparation of a microbubble-DNA mixture, followed by injection and ultrasound treatment of the target tissue;
- It allows highly efficient gene incorporation into mesenchymal tissues;
- It does not cause significant tissue damage-most sonicated chicken embryos survive without showing significant embryonic abnormalities or cell death;
- It can be used to introduce genes into several types of chicken embryo tissue (such as the branchial arch and lateral plate mesoderm).
Application of Sonoporation
Sonoporation can deliver genetic material to target organs by systemic administration while decreasing the associated risk of adverse effects, so it has been used for a variety of materials and in a variety of organs. To date, ultrasound-mediated gene delivery has been applied to heart, kidney, lung, muscle, blood vessel, brain, and many cancers with enhanced gene transfection efficiency, which depends on the ultrasound parameters such as acoustic pressure, pulse length, duty cycle, repetition rate, and exposure duration, as well as microbubble characteristics such as size, gas species, shell material, interfacial tension, and surface rigidity.
In vitro, a variety of cell lines have been successfully transfected with cell-killing effects. In vivo, initial applications have been used for cancer gene therapy, for which cell killing can be a useful simultaneous treatment. The drug delivery by sonoporation could enable the treatment of tumors with dense stroma such as pancreatic tumors, and has lower systemic toxicity than traditional chemotherapy. In addition, focused ultrasound-induced sonoporation is also an attractive option for the treatment of cardiovascular disease because it can be used in vivo and can greatly increase the specificity of treatments. Sonoporation has been used for non-viral gene delivery and has been used in a series of powerful in vitro and mammalian systems, which provides the basis and hope for the development of new gene therapy methods for clinical medicine.
Reference
- Du, X.; et al. (2018). Advanced physical techniques for gene delivery based on membrane perforation. Drug delivery. 25(1): 1516-1525. Distributed under Open Access license CC BY 4.0, without modification.
