Analytical Strategy of Biopharmaceutical Glycosylation
Importance of Glycan Analytics
Glycosylation is one of the most common post-translational modifications (PTMs) of proteins, present on more than 50% of the eukaryotic proteome. Glycosylation characterization of therapeutic proteins has become a regulatory requirement in pharmaceutical analytics, due to the potential effects on the activity and/or immunogenicity of the therapeutic proteins. Characterization of glycosylation includes the determination of the glycan sequences, linkage types, sites where glycans are attached to the protein, and macro- and micro-heterogeneity. Macro-heterogeneity is the heterogeneity of glycans which have variable percentages of occupancy of glycosylation site(s). Micro-heterogeneity is the heterogeneity of glycans that are attached to a specific glycosylation site.
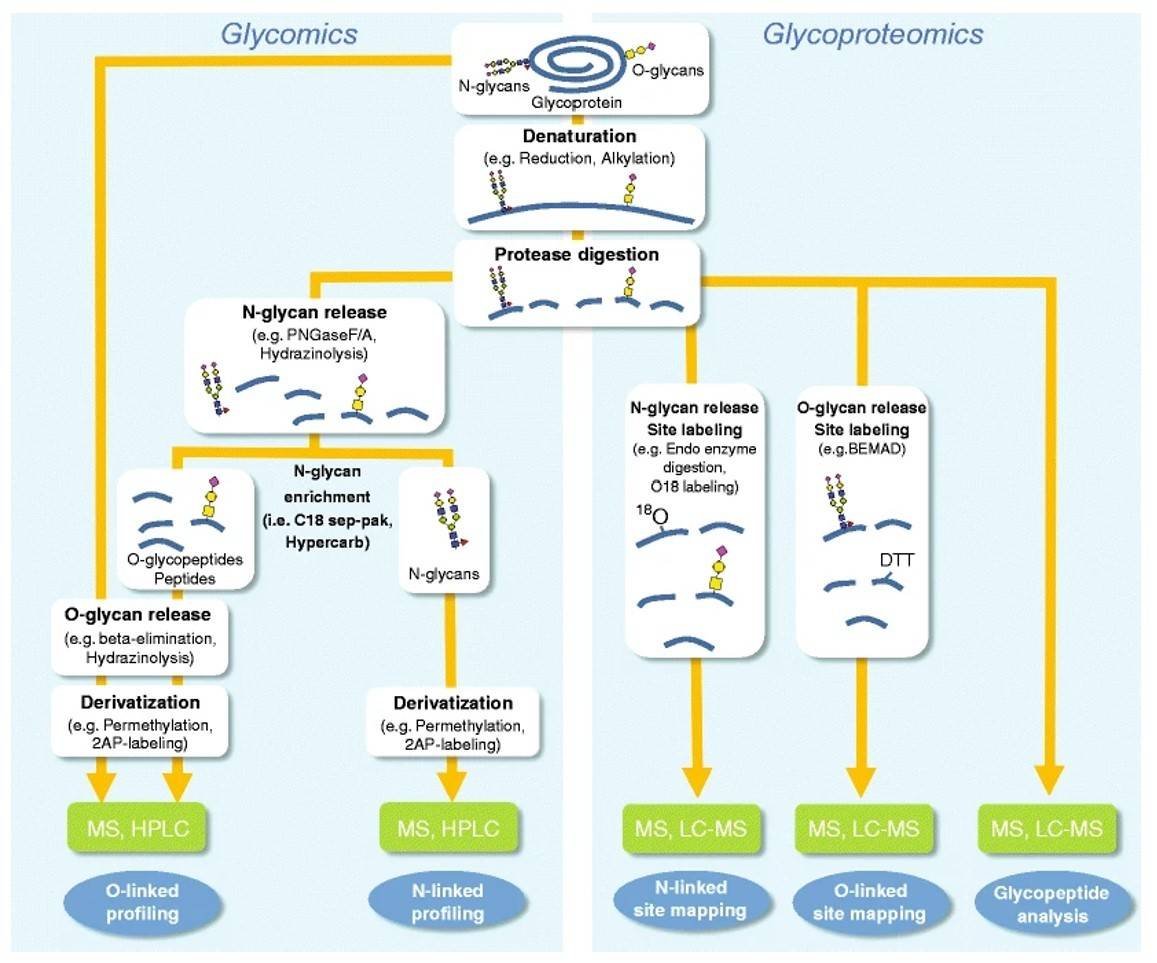 Fig.1 Flowchart for glycoprotein analysis.1, 3
Fig.1 Flowchart for glycoprotein analysis.1, 3
Methods for Glycan Analytics
-
Direct analysis of glycoforms
Direct analysis of glycoforms facilitates the analysis of glycosylation due to the minimal sample preparation. The development of such rapid methods is essential for the introduction of new glycoanalysis techniques to the pharmaceutical industry. For this purpose, capillary electrophoresis (CE) and liquid chromatography (LC) both coupled to high-resolution mass spectrometry (HR-MS) are largely used. CE offers excellent separation efficiency enabling the detection of individual intact glycoforms of glycoproteins, such as oxidized and acetylated variants of glycoforms. Accordingly, improvements in MS resolution facilitate the analysis of the intact molecules with higher accuracy.
Glycan mapping, or exact structural determination of glycans, is required for the release of batches of the therapeutic protein. The most straightforward and reproducible way to release N-glycans is digestion by peptide-N-glycosidase F (PNGase F) of unfolded protein. PNGase F catalyzes the cleavage between GlcNAc and asparagine amino acid residue. It releases almost all N-glycans from proteins except those bearing an α 1-3 linked fucose residue attached to the reducing terminal GlcNAc residue. In such cases, PNGase A can be used as an alternative. PNGase A shows lower efficiency in N-glycan release compared to PNGase F, but it releases N-glycans containing the α 1-3 linked fucose on the reducing terminal GlcNAc residue, an epitope that is produced by insect and plant cell lines. In certain cases, proteolytic digestion is recommended in order to improve the efficiency of the subsequent glycan release with PNGase F or PNGase A. However, PNGase F does not cleave glycans which are attached to the Asn residue in N- or C-terminal positions of a peptide. After glycan release, glycans are separated either by LC or by CE, which are coupled to appropriate detection instruments, including fluorescence and/or MS. Matrix-assisted laser desorption ionization time-of-flight MS (MALDI-TOF) analysis might also be used without any previous glycan separation and provide a mass-based fingerprint of glycans.
-
Determination of N-glycan macro- and micro-heterogeneity
Analysis of glycosylation macro- and micro-heterogeneity usually requires digestion of intact glycoproteins to glycopeptides. Glycoproteins are digested using specific proteases (e.g., trypsin, Lys-C, or pepsin, etc.). The resulting peptide mixtures are commonly analyzed by LC-HR-MS/MS with N-glycopeptide fragmentation. Tandem MS analysis resulting from collision-induced dissociation (CID) of N-linked glycopeptides has recently been proven to be very effective in the characterization of N-glycan microheterogeneity. Unfortunately, data processing, including MS/MS spectra of glycopeptides, is extremely time-consuming and requires high expertise of the analyst even where improvements to databases and software tools for interpretation have been made. As an alternative to CID, electron-transfer dissociation (ETD) tandem MS, which fragments the peptide backbone of glycopeptides leaving the modification intact enables easier identification of both the amino acid sequence of a glycopeptide and the unambiguous assignment of its glycosylation site.
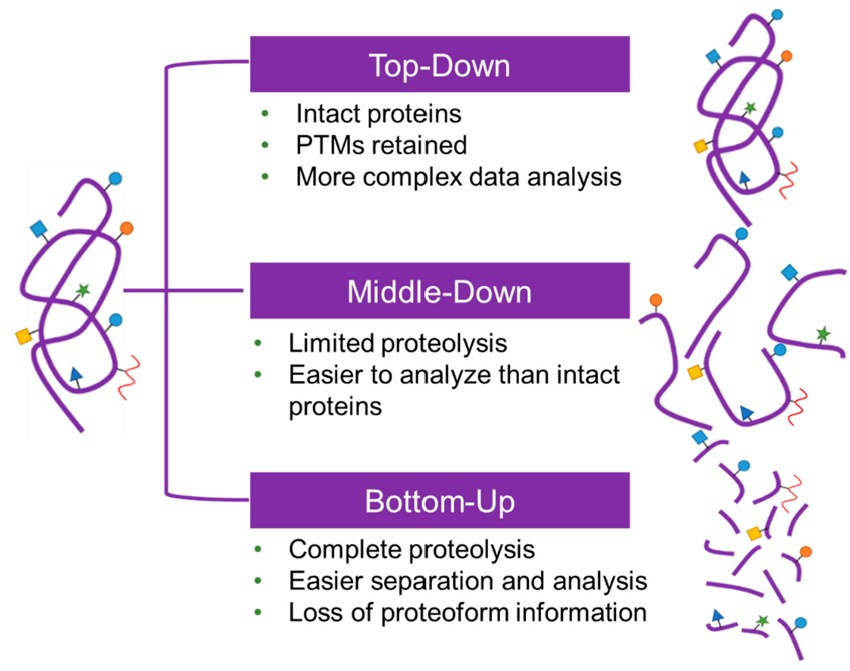 Fig.2 Procedure for analyzing glycoproteins by MS.2, 3
Fig.2 Procedure for analyzing glycoproteins by MS.2, 3
-
Analysis of monosaccharide content
Monosaccharide analysis is the simplest form of glycan characterization, enabling a determination of the type and the relative percentage of individual monosaccharides present in the complex glycan chains of therapeutic glycoproteins. For example, if sialylation, synthesis of complex glycans, or synthesis of high-mannose glycans was modulated, changes in the relative presence of monosaccharides will be detected. To facilitate monosaccharide analysis, N-glycans are hydrolyzed under acidic conditions. Obtained monosaccharides are later detected by anion-exchange chromatography (AEC) coupled to pulsed amperometric detection (PAD) or MS. PAD is mainly used for the analysis of nonacidic glycans and offers numerous advantages such as high sensitivity, baseline separation and avoidance of glycan derivatization, and associated sample clean-up. Monosaccharides can be also separated by capillary electrophoretic methods.
Services at Creative Biolabs
The importance of analysis for biopharmaceutical glycosylation is self-evident. Creative Biolabs has also developed a comprehensive platform to help our customers with glycoprotein analysis. Our glycoprotein analysis services include but are not limited to:
If you are looking for assistance in glycoprotein analysis, or you have any other questions about our services, please don't hesitate to contact us for more information.
References
-
Shajahan, Asif, et al. "Glycomic and glycoproteomic analysis of glycoproteins—a tutorial." Analytical and bioanalytical chemistry 409 (2017): 4483-4505.
-
Helms, Amanda, and Jennifer S. Brodbelt. "Mass Spectrometry Strategies for O-Glycoproteomics." Cells 13.5 (2024): 394.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Flowchart for glycoprotein analysis.1, 3
Fig.1 Flowchart for glycoprotein analysis.1, 3
 Fig.2 Procedure for analyzing glycoproteins by MS.2, 3
Fig.2 Procedure for analyzing glycoproteins by MS.2, 3

