Cell Line Glycoengineering Services
Protein glycosylation has already become a hot issue and nodus in glycoprotein expression and its application in biotherapeutic field. Manipulations and studies of glycosylation cannot be achieved by traditional genetic approaches. With years of experience
and first-in-class technologies, Creative Biolabs has successfully developed several effective strategies to provide a series of cell line glycoengineering services. In short, we have the capacity of producing glycoprotein with
human glycans in either research quantities or large scale for industrial applications.
Background of Cell Line Glycoengineering
Recombinant glycoproteins have shown different biological properties based on their glycan profiles. Monoclonal antibodies (mAbs) glycosylation is particularly important for pharmacokinetics and immunogenicity of recombinant glycoprotein therapeutics.
Thereby, different cell line glycoengineering strategies have been developed not only to improve cell's specific productivity but also to adapt their glycosylation profiles for increased therapeutic activity. Notably, the host cell line used to produce
glycoprotein has a strong influence on the glycosylation because different host systems may express various repertoire of glycosylation enzymes and transporters that contribute to specificity and heterogeneity in glycosylation profiles. Thus, cell
line glycoengineering is an essential process for the synthesis of desired glycoprotein no matter which production system you choose.
Features of Cell Line Glycoengineering Service
-
Different genetic approaches for manipulating glycosylation
-
Optional production system
-
Perfect after-sale service system
-
Therapeutic glycosylated IgGs with a higher ADCC function
-
Cost-effective and time-saving
With Ph.D. level scientists and over a decade of experience, Creative Biolabs has successfully established promising platforms for cell line glycoengineering and the production of glycosylated protein with improved characteristics
that are tailored to meet your R&D timeline and budget. Please contact us for more information and a detailed quote.
Published data
Glycosylation of therapeutic proteins significantly impacts their solubility, biological activity, and stability. However, most biologics still rely on CHO cell lines for production, which have relatively simple glycosylation characteristics but are similar to those of humans. Recently, scientists have focused on modifying insect, yeast, and plant cells to obtain glycosylation characteristics closer to humans. The main goal of glycoengineering CHO cells is to improve the consistency of glycosylation and enhance sialylation, thereby improving efficacy. Taking EPO as an example, it is an important glycoprotein, and its glycosylation state directly affects its efficacy and clearance rate. Therefore, it is of great significance to modify CHO cells by glycoengineering to achieve the production of non-fucosylated IgG antibodies. Although glycoengineering faces challenges such as local conformational effects, it provides a wide range of possibilities for the optimized design of glycoproteins. In addition, the advancement of gene editing technology has also provided new possibilities for studying glycosylation mechanisms and created new opportunities for further exploring the intracellular glycosylation process. In the future, genetic engineering of glycosylation will provide transformative opportunities for the development of recombinant glycoprotein therapies.
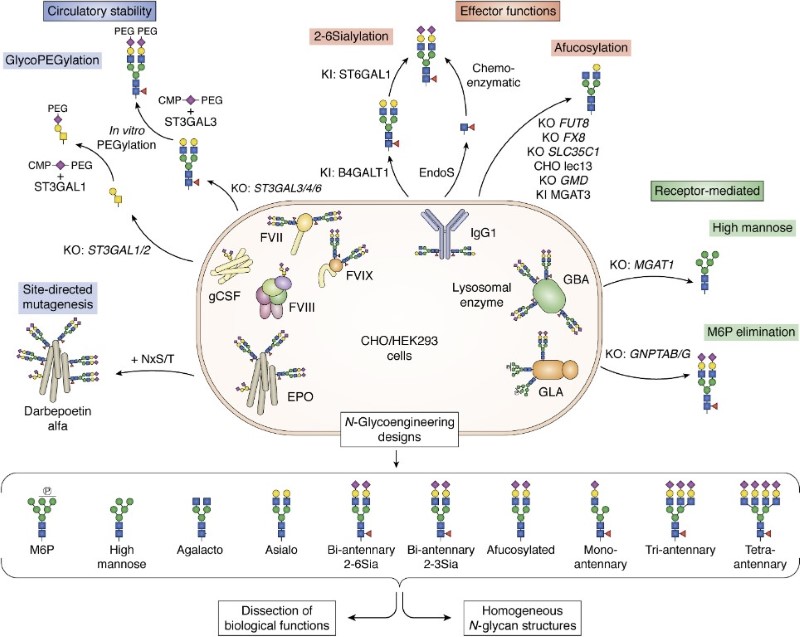 Fig.2 Glycoengineering strategies for recombinant glycoproteins.1
Fig.2 Glycoengineering strategies for recombinant glycoproteins.1
FAQs
Q1: How does glycoengineering impact the pharmacokinetics (PK) and efficacy of therapeutic proteins?
A1: Glycoengineering plays a pivotal role in modifying the pharmacokinetic properties of therapeutic proteins, influencing their absorption, distribution, metabolism, and excretion (ADME). A prime example of glycoengineering's impact can be observed with erythropoietin (EPO), where modifications in glycosylation have significantly enhanced its serum longevity and efficacy. By increasing the hydrodynamic radius of proteins through the addition of N-glycans, glycoengineering can reduce kidney filtration rates, thereby increasing the serum half-life of therapeutic proteins. This process directly impacts drug elimination, offering a pathway to enhance the efficacy of biologics by modulating their clearance rates from the body.
Q2: What are the latest advances in CRISPR technology for Cell Line Glycoengineering, and how do they address production and quality challenges?
A2: The advent of CRISPR technology has revolutionized Cell Line Glycoengineering, offering new avenues for enhancing protein production and improving product quality. Specifically, CRISPR has been utilized to modify the CHO (Chinese Hamster Ovary) cell lines, which are extensively used for producing biopharmaceuticals. Through targeted genome editing, CRISPR enables precise modulation of glycosylation patterns, augmentation of productivity, and elimination of problematic host cell proteins. Furthermore, CRISPR's ability to develop antibiotic-free selection systems and conduct site-specific transgene integration showcases its potential to address both production efficiency and quality control in biopharmaceutical manufacturing. This leap in technology not only ensures the safety and quality of the final products but also paves the way for more cost-effective development processes.
Customer Review
Transforming Therapeutic Protein Production
"As a biotech startup focused on developing novel therapeutic proteins, we faced significant challenges in protein glycosylation, which is crucial for the efficacy and safety of our products. Partnering with a Cell Line Glycoengineering Service was a game-changer for us. Their expertise in fine-tuning glycosylation patterns using advanced CRISPR technology allowed us to achieve human-like glycosylation profiles, which significantly enhanced our product's biological activity and serum half-life. The team's professionalism, coupled with their cutting-edge techniques, not only accelerated our R&D timeline but also ensured that our proteins met stringent regulatory standards. It's been a truly transformative experience for our product development efforts."
Elevating Product Quality with Precision Glycoengineering
"In the competitive field of monoclonal antibody production, the quality and consistency of glycosylation can make or break a product. That's why we turned to a leading Cell Line Glycoengineering Service to address this critical aspect. Their comprehensive services, from glycosylation analysis to cell line modification, provided us with unparalleled insights and solutions to optimize our antibody products. The results were outstanding: improved ADCC activity, enhanced stability, and reduced immunogenicity. Their dedicated scientific team worked closely with us throughout the process, ensuring our specific needs were met with precision and care. Our investment in their glycoengineering services has paid dividends in product quality and patient outcomes."
Reference
-
Narimatsu, Yoshiki, et al. "Genetic glycoengineering in mammalian cells." Journal of Biological Chemistry 296 (2021). Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

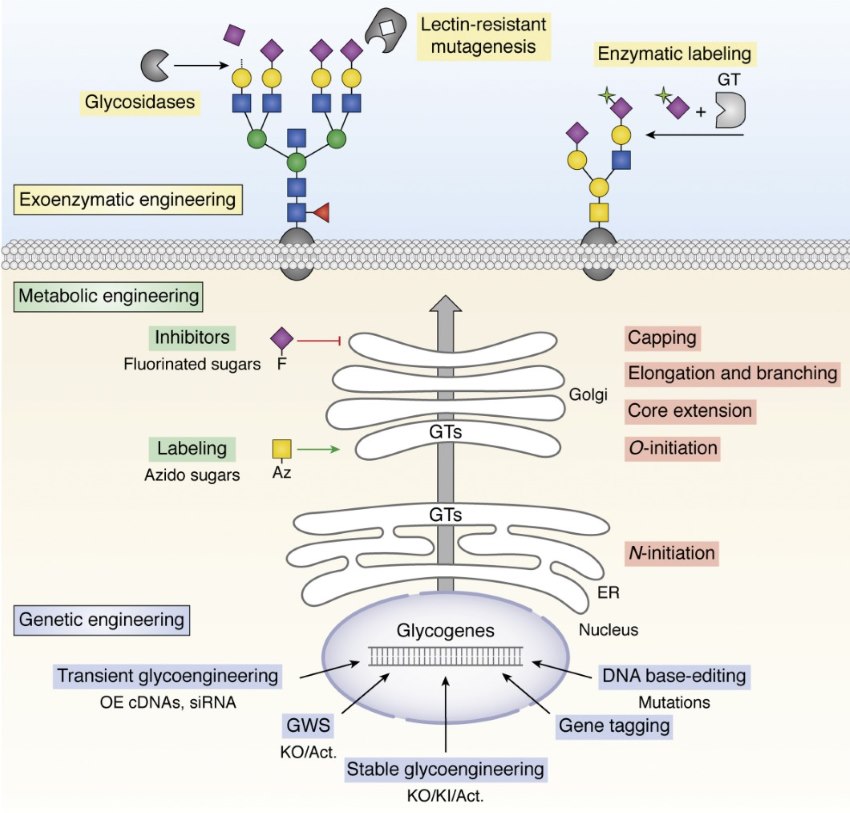 Fig.1 Genetic glycoengineering-based cellular glycosylation strategy.1
Fig.1 Genetic glycoengineering-based cellular glycosylation strategy.1
 Fig.2 Glycoengineering strategies for recombinant glycoproteins.1
Fig.2 Glycoengineering strategies for recombinant glycoproteins.1

