Glycan Labeling
Introduction of Glycan Labeling
Glycan analysis has been widely used in multiple fields, including biological basic research, diagnosis application, and clinical analysis. In general, a glycan derivatization step is always required for the introduction of a chromophore or fluorophore.
Reductive amination, Michael addition, and hydrazide labeling are the most commonly used reactions that require the reducing end of the glycan. To avoid the excessive presence of labeling reagents, analysis techniques such as size exclusion chromatography and solid-phase extraction (SPE) are used to remove excess labeling reagents from the samples. For the structural characterization of glycans, permethylation can be used to reduce the reducing terminal aldose by sodium borohydride to obtain the ring-opened alditol, thereby providing a quality label that is helpful for spectral interpretation.
Functions of Derivatization Strategies
-
Enhance glycan separation
-
Improve the stability of sialic acid residues
-
Improve the sensitivity of mass spectrometry
-
The detailed structure can be characterized by (tandem) MS
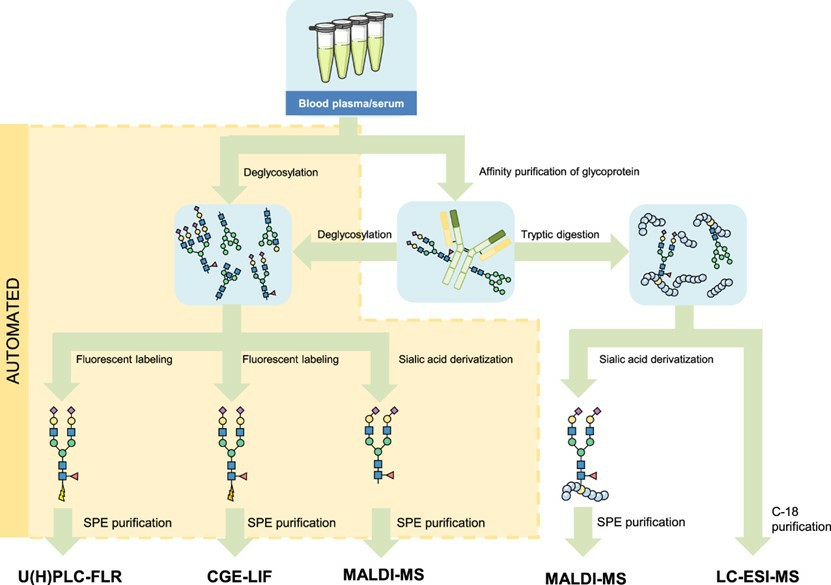 Fig.1 Labeling and analysis strategies for glycomics.1
Fig.1 Labeling and analysis strategies for glycomics.1
Reductive Amination
Reductive amination is the method to label glycans at their reducing end. In this reaction, the label contains a primary amine group that reacts with the aldehyde group of the glycan, resulting in an imine or Schiff base, which is then reduced to a secondary amine. The advantage of reductive amination is that one label is attached to each glycan stoichiometry, allowing direct quantification based on fluorescence or UV absorption intensity. The commonly used labels include 2-aminobenzamide (2-AB), 2-aminobenzoic acid (2-AA), 2-aminopyridine (PA), 2-aminonaphthalene trisulfonic acid (ANTS), and 1-aminopyrene-3,6,8-trisulfonic acid (APTS).
Michael Addition
Michael addition is the approach that carbanion forms a nucleophilic addition with α,β-unsaturated carbonyl compounds. It is one of the most common methods for the mild formation of C–C bonds under alkaline conditions. 1-phenyl-3-methyl-5-pyrazolone (PMP) and its methoxy analog 1-(p-methoxy)-phenyl-3-methyl-5-pyrazolone are important labeling reagents. However, the use of the PMP label is largely limited as there are no fluorescent labels reported.
Hydrazide Labeling
Hydrazide labeling is the process that labels hydrazide end groups on glycans. Hydrazide labeling has been widely used for mass-spectrometric detection, chromatography, as well as biomolecular interaction analysis. Carboxymethyl trimethylammonium hydrazide is a label that places a permanent positive charge at the reducing end of a glycan for sensitive analysis via MALDI time of flight (TOF) MS.
(Per-)methylation
Permethylation is an advantageous derivatization approach. The hydrogens on the amine, hydroxyl, and carboxyl groups are replaced by methyl groups. This process enhances the signal intensity of polysaccharides in MS through ESI and MALDI, which facilitates spectral analysis. What’s more, detailed information related to linkage positions can be obtained through tandem MS of permethylated glycans. For the structural characterization of glycans, permethylation allows the reduction of the reducing terminal aldose by sodium borohydride to obtain the ring-opened alditol, thereby providing a mass label for spectral interpretation.
Creative Biolabs has been a long-term expert in the field of glycomics research. Now we offer a variety of products and services including glycan modification and labeling. If you are interested in our products or services, please do not hesitate to contact us for more detailed information.
Reference
-
Trbojević-Akmačić, Irena, et al. "High-throughput glycomic methods." Chemical reviews 122.20 (2022): 15865-15913. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Labeling and analysis strategies for glycomics.1
Fig.1 Labeling and analysis strategies for glycomics.1

