Glycan Profiling Service
Glycoproteins are involved in a variety of biological processes, but glycoprotein analysis is a big challenge because of the low abundance and heterogeneity of glycans. As a forward-looking company as well as a leading customer service provider, Creative Biolabs has successfully explored innovative and versatile glycoprotein analysis platforms to provide diverse portfolio of glycoprotein structure analysis services. Glycan profiling is an important part of glycoprotein structure analysis we provide and glycan profiling service has helped us win a good reputation among our clients. We are glad to help you get milestone success in your glycoprotein project.
Glycan Profiling Is Needed for Glycoprotein Structure Analysis
As a fundamental post-translational modification of proteins, glycosylation plays an important role in many physiological and pathological processes and is involved in multiple diseases, including cancer and autoimmune diseases as well as in congenital disorders of glycosylation. Glycoprotein structure analysis allows characterization of important biological molecules and would be of great diagnostic and clinical importance. Glycan profiling is an important part of glycoprotein structure analysis. As a novel approach to the structural analysis of glycans, glycan profiling put the emphasis on capturing essential information of target glycans in a rapid, sensitive, and high-throughput manner rather than defining covalent structures in a rigorous and time-consuming manner. Glycan profiling is particularly important for structural glycomics with the absence of a usefully automated sequencer analogous to protein and DNA sequencers. N-glycan and O-glycan profiling are two common types of analysis.
N-Glycan Profiling in Creative Biolabs
N-linked oligosaccharide is usually attached to the asparagine residue and N-glycosylation occurs solely on proteins that shuttle via the secretory pathway. With the increasing application of protein therapeutics, such as monoclonal antibodies, glycoproteins, hormones, cytokines and clotting factors in clinical practice, routinely N-glycan profiling has gained increasing scientific interest. At present, N-glycan profiling has been widely used in the identification of various diseases, including cancer, Alzheimer’s, and diabetes. Technologies for N-Glycan profiling in Creative Biolabs including but not limited to:
-
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) is a high-throughput method to analyze the structural characterization of glycosylated compounds with high sensitivity and robustness. It also prefers to profile mixtures of N-glycans due to the preference for singly charged ion formation that makes MALDI a better choice than electrospray ionization. During the detection, N-glycans are released by enzymatic deglycosylation (Peptide N-glycosidase A or F) or chemical methods such as Beta-elimination and hydrazinolysis, and then, they are analyzed after permethylation.
-
Ultra-high performance liquid chromatography linked with fluorescence detection/mass spectrometry (UPLC-FLD/MS) has been a powerful technology for N-glycan profiling. Glycoprofiling of enzymatic glycan release with PNGase F followed by derivatization by 2-aminobenzamide (2-AB) is a fast and robust technique to analyze larger numbers of samples with well-known N-glycan profiles.
-
High-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) is a sensitive and specific analytical method for the monosaccharide composition analysis. This direct analysis allows individual glycan monosaccharides analysis, providing not only the total glycosylation of a protein but also the amounts of specific monosaccharides.
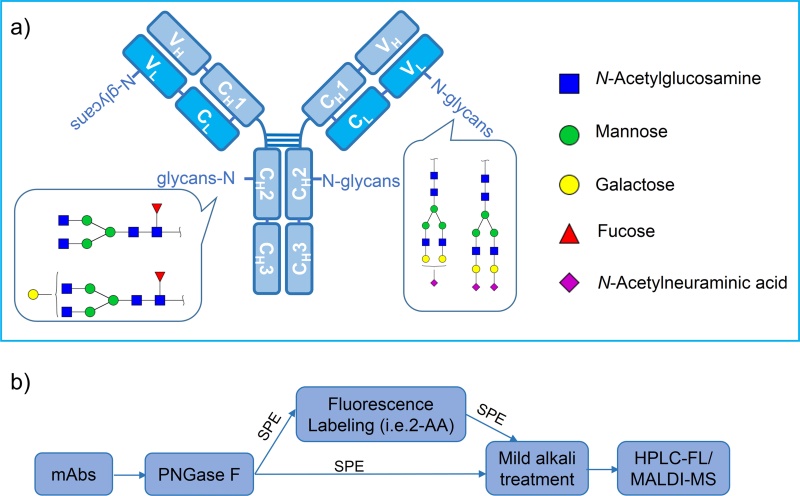 Fig.1 Antibody structure and N-glycosylation analysis process.1, 3
Fig.1 Antibody structure and N-glycosylation analysis process.1, 3
O-Glycan Profiling in Creative Biolabs
O-acetylgalactosamine (O-GalNAc) and O-acetylglucosamine (O-GlcNAc) are the common O-glycosylations types, among which, O-GalNAc is attached to the hydroxyl group of the protein serine or threonine residues through an α-linkage, while O-GlcNAc is attached through a β-linkage. Unlike N-glycans, there is no known O-linked amino acid consensus sequence yet.
Technologies for O-Glycan Profiling in Creative Biolabs including but not limited to:
-
MALDI-TOF MS is the most common method for O-glycans profiling. Before O-glycans profiling, permethylation of the O-glycan to transform all hydroxyl groups into methyl ethers and stabilizes sialic acids by methyl esterification of their carboxyl groups is necessary, which allows MALDI-TOF MS to analyze of all O-glycans in the positive mode. Direct infusion of O-glycans of permethylation into the mass spectrometer can provide comprehensive information about glycans composition.
-
HPAEC-PAD can also provide details about fucosylation, which can impact protein function and signaling, gives an indication of the presence of O-linked glycans.
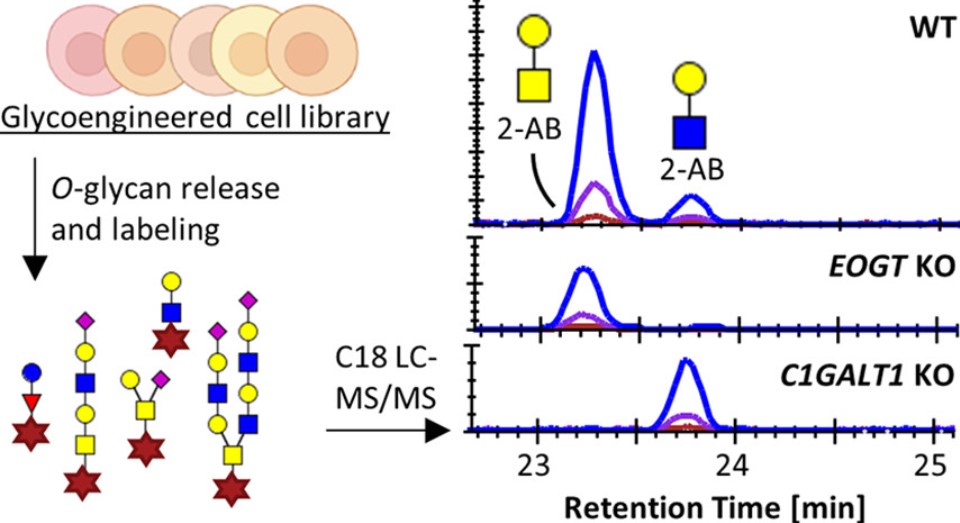 Fig.2 O-Glycan profiling.2, 3
Fig.2 O-Glycan profiling.2, 3
Features of Our Glycan Profiling Service
-
High sensitivity and specificity
-
Stability and consistency
-
Competitive prices with quality service
-
Best after-sale service
Working with Us to Promote Your Success!
Glycan profiling has become a regular study in glycosylation engineering, which is beneficial for the assignment of distinct functional properties to defined structural features. With years of experience, Creative Biolabs has successfully completed a lot of glycan profiling projects. We can offer a whole set of glycoprotein structure analysis service to help you get landmark development. We can also customize our offering to meet your specific project needs. If you are interested, please contact us without hesitation.
Published data
Mature O-glycans are composed of a variety of polysaccharide chains with rich structural variations and branching characteristics. O-glycosylation is a common modification in the proteome that affects the functions of a variety of cells. In this study, the authors developed a high-throughput sample preparation technology that can release O-glycans from proteins in cell lysates in a minimally destructive, non-reducing manner. The reducing ends of the released O-glycans were labeled with 2-AB, which effectively facilitated the separation and detection of isomers. In addition, the authors combined this method with some glycoengineered cell lines to achieve characteristic structural analysis of different types of O-glycans and their O-GalNAc core extensions. This method was applied to a variety of sample types and provided new opportunities for studying glycomic changes in human tissues and disease models.
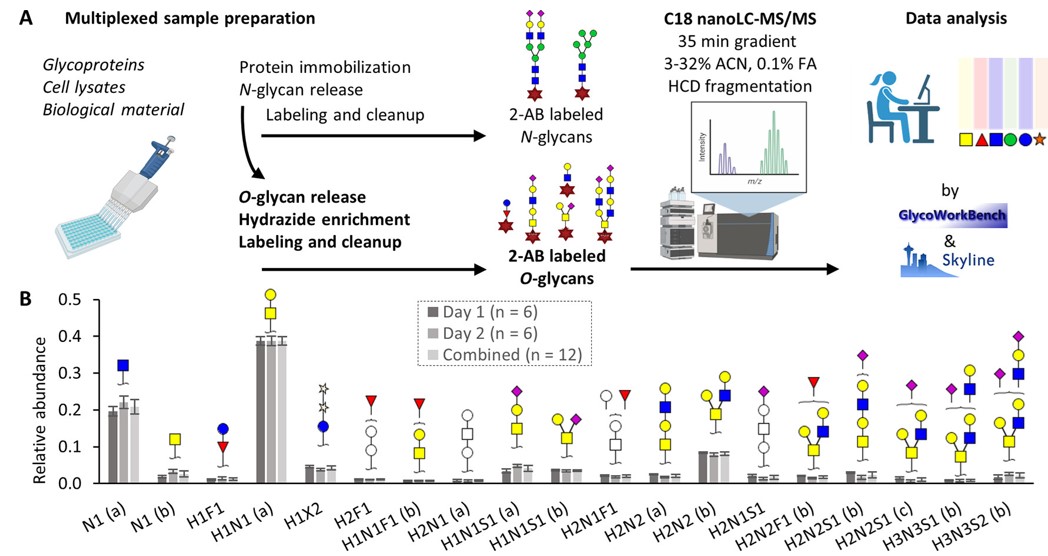 Fig.3 Workflow of preparation and analysis of O-glycans.2, 3
Fig.3 Workflow of preparation and analysis of O-glycans.2, 3
Case Study
Background: The client commissioned Creative Biolabs to perform N-glycan analysis on 9 antibody samples.
Method: Fluorescence detection-liquid chromatography-mass spectrometry (FLD-LC-MS)
Abstract: The client provided us with 9 antibody samples, labeled as sample A, sample B, sample C, sample D, sample E, sample F, sample G, sample H, and Sample_Azide. We were commissioned to obtain an N-glycan analysis of these 9 samples and the N-glycan structure mapping of Sample_Azide.
We mixed 15 μg of glycoprotein with 6 μL of 5% (w/v) RapiGest SF solution and heated it to 90°C for 3 minutes. 1.2 μL of PNGase-F enzyme was added to release glycans, and after the sample cooled, it was incubated at 50°C for 5 minutes.
We added 12 μL of labeling reagent (RapiFluor-MS) to the deglycosylation mixture and used a micropipette to aspirate and dispense the solution to mix. The reaction was allowed to proceed at room temperature for 5 minutes and then acetonitrile was added to stop the reaction.
The GlycoWorks HILIC µElution plate was equilibrated with MilliQ water and then with ACN: H2O (85:15). The samples were added to the wells and washed with formic acid: H2O: ACN (1:9:90). Finally, the glycans were eluted with 200 mM ammonium acetate in 5% acetonitrile.
We selected a GlycanPac AXH-1, 2.7 μm, 2.1×150mm column for glycan analysis by FLD-LC-MS and searched the detected mass against the internal database for glycan structure identification. For the Sample_Azide sample, ~70% N-glycans were G0f-HexNAz2 and G0f-GN-HexNAz. The figure below was the MS analysis result of G0f-HexNAz.
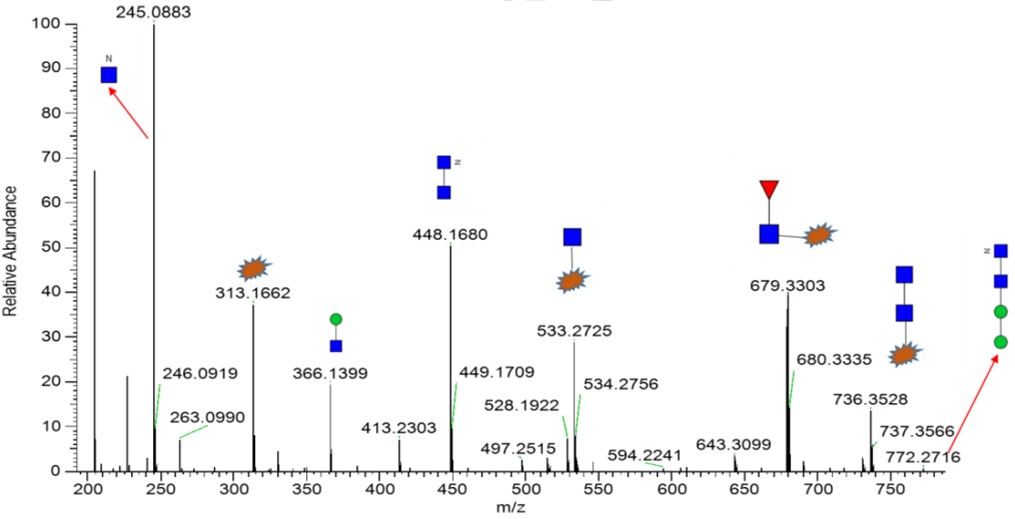 Fig.4 MS/MS profile of G0f-HexNAz.
Fig.4 MS/MS profile of G0f-HexNAz.
FAQ
Q1: What are the advantages of MALDI-TOF MS in glycan analysis?
A1: MALDI-TOF MS has the advantages of high sensitivity, fast data acquisition, and excellent resolution in N- and O-glycan analysis. It effectively detects low-abundance glycan molecules and adapts to the direct analysis of complex samples. Moreover, it processes multiple glycan molecules at the same time, simplifies the pretreatment steps, provides clear spectra for glycans with similar structures, and helps to deeply understand the role of glycosylation in biological processes.
Q2: How to choose the right glycan analysis technology?
A2: When choosing the right N- and O-glycan analysis technology, the characteristics of the sample and the purpose of the study should be considered first. If you are concerned about the structure and function relationship of sugar chains, it is recommended to use mass spectrometry (MS) combined with liquid chromatography (LC), which can provide high resolution and quantitative capabilities. If you need to detect low-abundance glycan molecules, MALDI-TOF MS may be the best choice, while for fast and high-resolution analysis, UPLC-FLD/MS is more suitable. On this basis, we provide professional advice based on specific needs to ensure that the most appropriate method is selected.
Q3: What kind of samples do I need to provide for glycan analysis?
A3: When performing glycan analysis, you will need to provide purified glycoprotein samples, preferably in a properly extracted and concentrated form to ensure the integrity and stability of the glycans in the sample. In addition, the sample should be free of contamination and stored under proper conditions to prevent degradation or glycosylation changes. The specific sample volume and processing requirements can be further confirmed according to the selected analytical technique, and we recommend that you communicate with us for detailed guidance.
Customer Review
Efficient Workflow
“Creative Biolabs' N-glycan analysis service provides a clear and efficient workflow. The time from sample submission to results is greatly shortened, which greatly improves our research efficiency. Creative Biolabs has a good reputation in the industry. We chose them because of previous client recommendations, and now it seems that we are not disappointed at all.”
Comprehensive Data Interpretation
“Creative Biolabs' scientists not only provide data results of glycan analysis but also provide detailed interpretations, which enable us to better understand the impact of glycosylation on the development of cancer, which provides new perspectives for our clinical research.”
References
-
Liu, Sheng, et al. "Comprehensive N-glycan profiling of cetuximab biosimilar candidate by NP-HPLC and MALDI-MS." PLoS One 12.1 (2017): e0170013.
-
de Haan, Noortje, et al. "In-depth profiling of O-glycan isomers in human cells using C18 nanoliquid chromatography–mass spectrometry and glycogenomics." Analytical Chemistry 94.10 (2022): 4343-4351.
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 Antibody structure and N-glycosylation analysis process.1, 3
Fig.1 Antibody structure and N-glycosylation analysis process.1, 3
 Fig.2 O-Glycan profiling.2, 3
Fig.2 O-Glycan profiling.2, 3
 Fig.3 Workflow of preparation and analysis of O-glycans.2, 3
Fig.3 Workflow of preparation and analysis of O-glycans.2, 3
 Fig.4 MS/MS profile of G0f-HexNAz.
Fig.4 MS/MS profile of G0f-HexNAz.

