Glyco-engineered Mammalian Cell Expression System
The mammalian cell expression system is by far the most used production platform for glycoproteins production. Production of glycoproteins is currently achieved by either transient or stable gene expression in the cells. Although the level of protein obtained by transient expression is not as high as with stable gene expression, it is still sufficient for many applications. When it comes to the production of glycoproteins in large quantities, stable gene expression systems remain the preferred avenue.
With years of research and experience in mammalian cell glycoengineering, multiple adverse factors have been tackled and optimized for improving the productivity of glycoprotein, process robustness, and reducing cell line generation timelines. Creative Biolabs has successfully developed our own glyco-engineered Chinese hamster ovary (CHO) cells and glyco-engineered human embryonic kidney 293 (HEK293) cells for glycoprotein production. We can offer high-quality customized services by adjusting strategies to meet even the most specific requirements in the production of glycoprotein.
Mammalian System for Glycoproteins Production
A significant portion of biopharmaceutical products are recombinant proteins, with an ongoing increase in the number of them produced in mammalian expression platforms. This trend is mostly driven by the increased attention directed to post-translational modifications (PTM) of these biologics, in particular towards their glycosylation state. Scientists have made great efforts to understand how glycosylation can influence the biological activity of therapeutics. Studies have shown that proper glycosylation can improve protein properties, such as high stability, the long half-life, and low immunogenicity, etc.
CHO
Among the mammalian systems, CHO cell is by far, the most commonly used cell line. It is involved in the production of over 70% of recombinant biopharmaceutical proteins. CHO system is widely used for glycoprotein production due to numerous advantages. 1) Different gene expression systems have been developed and used in CHO cells, which allow for high titer yields and good specific productivity. 2) CHO cells can achieve a substantial production rate and can be adapted to grow in various serum-free and chemically defined culture media. Thus, this system is suitable for large-scale industrial suspension culture. 3) CHO system can produce glycoproteins with human-like glycans, making the generated products more likely to be compatible and bioactive within human hosts. 3) Moreover, CHO cell is refractory to infection by human viruses, which minimizes biosafety risks.
HEK293
One way to favor human-like glycosylation would be to use human cell lines for recombinant protein production. This strategy would warrant that proteins harbor, if not the ideal glycosylation pattern, at least a non-immunogenic glycans. HEK293 is the most commonly used human cell lines to manufacture glycoprotein therapeutics are the HEK293 cells.
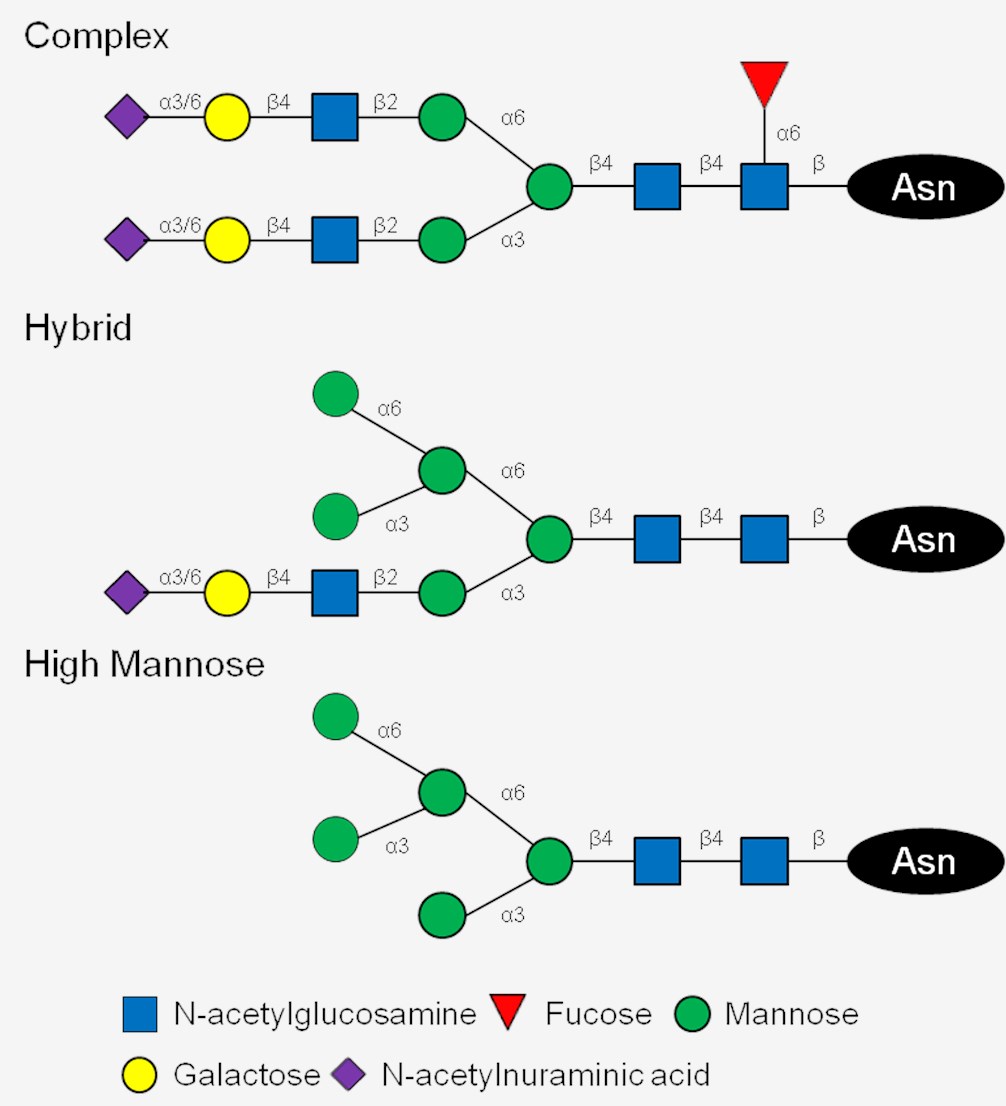 Fig.1 Major N-linked glycans in mammalian cells.1
Fig.1 Major N-linked glycans in mammalian cells.1
Glyco-engineered Mammalian Cell for Glycoprotein Production at Creative Biolabs
Creative Biolabs has engineered CHO system and HEK 293 system for glycoprotein production. Those systems can even ensure the natural folding and sugar chain composition of glycoprotein, and significantly increase the expression level of glycoprotein, and fundamentally solve the problem of humanization of recombinant glycoprotein.
Features of Our Technology
-
An in-house highly efficient expression vectors
The signal peptide in the vector is an artificial signal peptide with the strongest secretion ability derived by bioinformatics, which greatly promotes the production of secreted proteins. Genetic element has been added in upstream of the promoter and downstream of the poly A to protect the target gene and shield gene silencing, which is a major limiting factor in obtaining highly expressing cell lines.
-
Improved cell line with high expression of recombinant glycoprotein
In addition to a mature and efficient expression vector, we use gene-editing technology to insert glycosylated elements or knock out glycosylated genes to modify the host CHO cells and HEK 293 cells to improve the glycosylation of the target protein.
-
Optimized protein expression strategies
Our technical team has rich experience in CHO cell and HEK 293 cell expression systems. We are dedicated to serving as your one-stop-shop for glycoprotein production, from codon optimization, selecting the appropriate signal peptide, choosing the appropriate transfection and screening methods, improving the success rate and yield of glycoprotein expression, etc.
Highlights
-
One-stop service: From vector construction to the purification of glycoprotein.
-
Qualified cell lines: Guarantee the quality of glycoprotein expression.
-
High success rate: Multiple glycoproteins have been successfully produced in our mammalian expression system.
-
High-quality glycoprotein: Affinity purification, dialysis, and ultrafiltration are used to maximize the activity of glycoprotein.
-
High yield of glycoprotein expression level.
-
Large-scale production: Several grams of a glycoprotein can be produced through suspension culture.
-
Solving the problem of low expression of glycoproteins: Optimizing gene sequence codons to increase glycoprotein expression; selecting the most appropriate signal peptides to secrete glycoproteins outside the cell to reduce the impact on normal physiological activities of cells; reducing the aggregation of glycoprotein by introducing gene mutation through the bioinformatics strategy.
Creative Biolabs is a leading service provider that focuses on developing glycoproteins and glycosylated monoclonal antibodies with high-specificity and high-affinity for research, diagnostic, and therapeutic use. We own qualified mammalian cell lines for the development of glycoprotein. If you are interested in glycoprotein production, please contact us for more information and a detailed quote.
Published data
Mammalian cell expression systems are efficient platforms for glycoprotein production. This paper introduces us to the glycosylation pathway and the major biosynthetic steps. Various glycosyltransferases play a role in glycosylation in mammalian cells and are involved in the assembly of various glycan structures. Rational engineering of glycosylation in mammalian cells is often required to produce specific glycoproteins. The development of gene editing techniques has made it possible to accurately regulate the glycosylation pathway in mammalian cells. In addition to the glycosylation pathway, this paper also introduces us to genome editing techniques for constructing expression systems in mammalian cells. By understanding glycosylation pathways and gene editing technologies, we can better rationally design glycosylation pathways in mammalian cells, screen gene editing technologies, and construct mammalian cell expression systems.
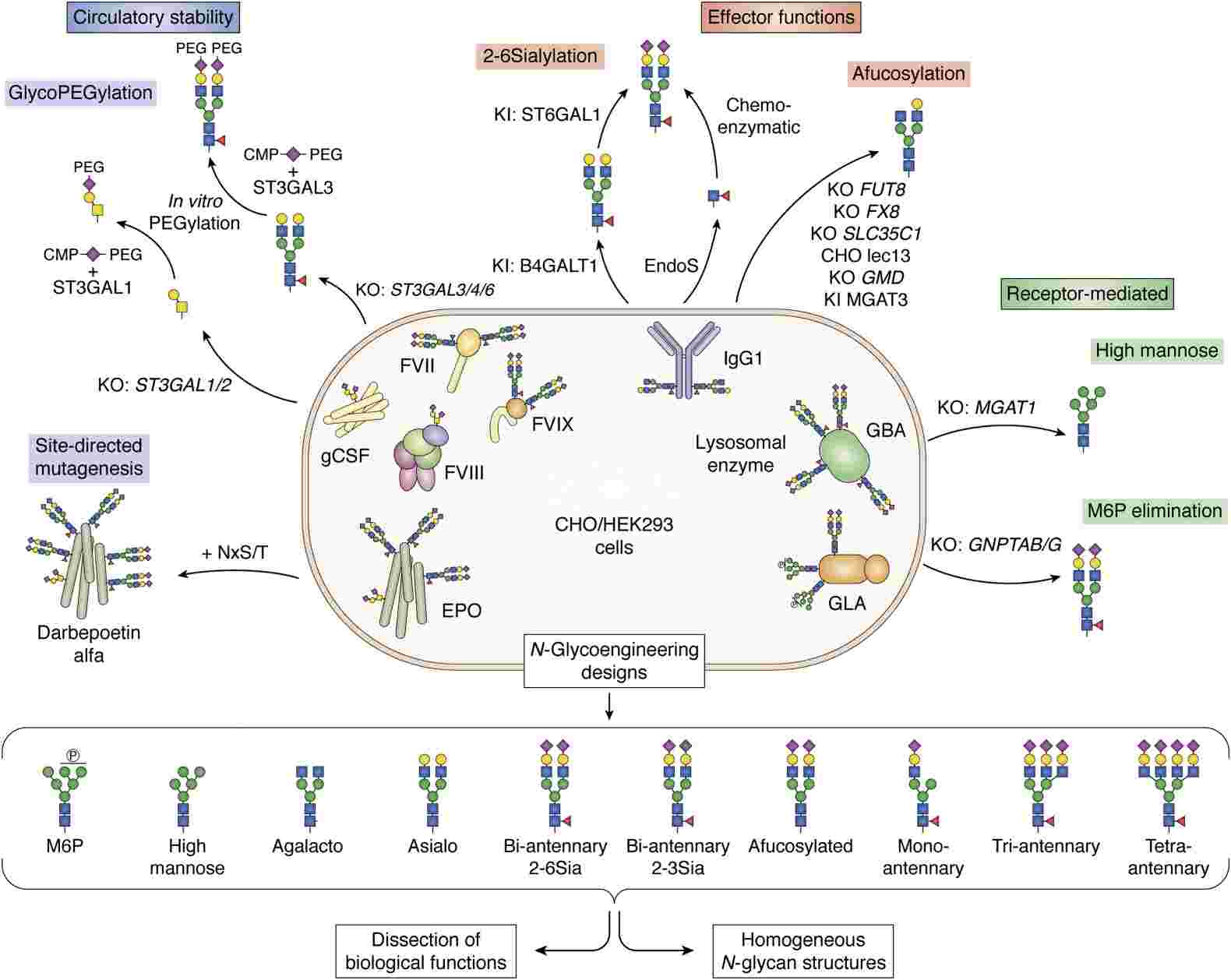 Fig.2 Glycoengineering strategies for therapeutic glycoproteins.2
Fig.2 Glycoengineering strategies for therapeutic glycoproteins.2
FAQs
Q1: What are the advantages of glyco-engineered mammalian cell expression systems?
A1: The mammalian cell expression system offers several advantages in the production of therapeutic glycoproteins, including the following:
-
Human-like, relatively simple, and inherent glycosylation capabilities.
-
Relatively high productivity.
-
Good bioactivity and biocompatibility of products.
-
Reduced biosafety risks.
Q2: What types of therapeutic glycoproteins are suitable for production by glyco-engineered mammalian cell expression systems?
A2: A wide range of therapeutic proteins, such as monoclonal antibodies, enzymes, hormones, blood factors, etc., is produced using glyco-engineered mammalian cell expression systems.
Q3: What are the advantages of glyco-engineered mammalian cell expression systems over yeast and bacterial systems for the production of therapeutic glycoproteins?
A3: Glycosylation of therapeutic glycoproteins may affect their stability, solubility, structure, cellular uptake, and biological activity. Glycoengineered mammalian cell systems offer more human-like glycosylation patterns than bacterial or yeast systems and are better suited for the production of complex glycoproteins with complex glycan structures.
Customer Review
A Deep Understanding of Mammalian Cell Glycosylation
"We recently commissioned Creative Biolabs to help us construct a mammalian cell expression system for the production of therapeutic glycoproteins. From the initial communication to the conduct of the experiment, the whole process went very smoothly. With their deep understanding of glycosylation, they significantly increased the expression levels of glycoproteins. There is no doubt that they are experts in the production of therapeutic glycoproteins using mammalian cell expression systems."
Improved Production of Therapeutic Glycoproteins
"Creative Biolabs had an in-depth understanding of the engineering of glycosylation and glycoproteins in mammalian cells. After understanding our specific requirements and goals, they tailored unparalleled solutions and optimized our products. They not only delivered on their promise of quality for the glyco-engineered mammalian cell expression system but also provided valuable insights and guidance. The collaboration was a great experience."
References
-
Nettleship, Joanne E. "Structural biology of glycoproteins." Glycosylation. InTech, London (2012): 41-62. Distributed under Open Access license CC BY 3.0, without modification.
-
Narimatsu, Yoshiki, et al. "Genetic glycoengineering in mammalian cells." Journal of Biological Chemistry 296 (2021). Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 Major N-linked glycans in mammalian cells.1
Fig.1 Major N-linked glycans in mammalian cells.1
 Fig.2 Glycoengineering strategies for therapeutic glycoproteins.2
Fig.2 Glycoengineering strategies for therapeutic glycoproteins.2

